In Part One we looked at various aspects of how our intestinal microbiota was affected by the food we put in our mouths, particularly with regard to variations that occur between plant- and meat-based diets and in relation to the macronutrient, carbohydrate. In Part Two, we’ll take a look at the microbial effects of the other two macronutrients:
- protein, and
- fat
as well as the following:
- polyphenols
- postbiotics
- SCFAs 1
- phytoestrogens
- isothiocyanates
- aryl-hydrocarbon receptor ligands
- Coprostanol and secondary bile acids
- trimethylamine N-oxide (TMAO), and
- vitamins
Protein
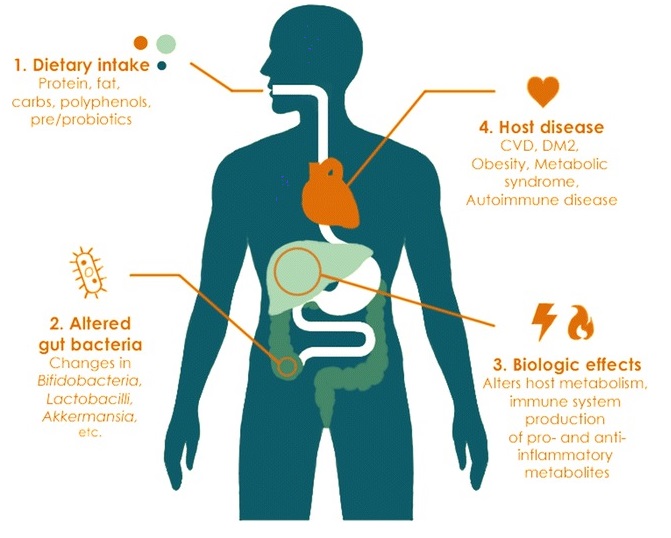
In previous blogs 2 3 4 5 , we’ve looked in great detail at the effects of protein (both animal and plant) on human health. Studies mentioned in the latter blogs have shown how consumption of animal protein (especially in large quantities) is associated with a wide range of common diseases.
When it comes to the effects of protein on gut microbes, the majority of studies suggest 6 that plant protein consumption has a strongly positive correlation with improved healthy microbial diversity.
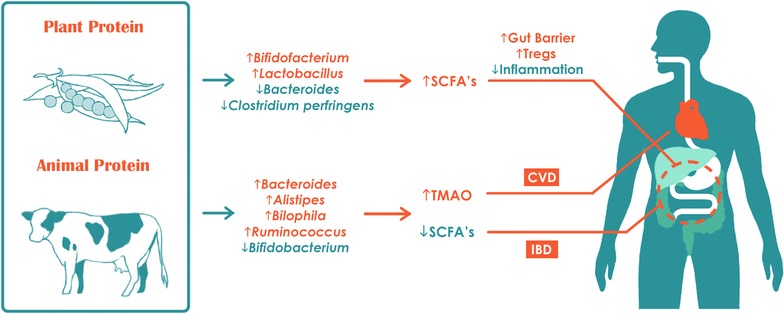
The following bacteria are commonly increased in number within the gut microbiota of those individuals consuming a high animal protein diet:
The latter are bile-tolerant microbes 11 . Bile increases when animal-protein consumption increases, when compared with increased plant-protein consumption, so it’s no surprise that a meat-based diet will mean that bile-tolerant microorganisms will increase in number 12 .
On the other hand, the following bacteria are commonly decreased in number within the gut microbiota of those individuals consuming a high animal protein diet:
The latter are important for metabolising dietary plant polysaccharides 16 . Again, it’s no surprise that these plant polysaccharide-loving bacteria will frequent the guts of plant-eaters.
It’s a zero-sum game
Another factor which needs to be taken into account is that diet is a zero-sum 17 game: the more protein in your diet, the less room there is for healthy plant carbohydrate. The result of this will be a decrease in butyrate-producing bacteria, and thus an increase in the proinflammatory bodily state and an increased risk of colorectal cancer 18 .
When individuals have eaten pea protein, for instance, it’s been shown 6 that there is a corresponding increase in beneficial Bifidobacterium and Lactobacillus, while the pathogenic Bacteroides fragilis and Clostridium perfringens reduce. The result of this is that there is an increase in intestinal SCFA levels (more on SCFAs below). The latter study drew the conclusion that eating plant-derived proteins reduces mortality when compared with eating animal-derived proteins.
Fats
Both quantity and quality of consumed fat have been shown 18 to have significant impact on the composition of gut microbiota.
When you eat a plant-based diet (and here we’re talking about whole, unprocessed plant foods), it will be naturally low in fat. This favours the beneficial Bifidobacteria 19 .
Plant-based fats
The fats from a plant-based diet are made up of mainly mono and polyunsaturated fats. On a phyla level, the result of this is that the Bacteroidetes:Firmicutes ratio 20 increases, while on the genera level, lactic acid bacteria (Bifidobacteria and Akkermansia muciniphila) increase 6 .
Nuts about gut bacteria

Previous blogs 21 looked at how walnuts are particularly good plant-based sources of omega-3 fatty acids (the ALA within them being converted to DHA and EPA within our bodies); however, it doesn’t stop there. Walnuts, and other nuts in general, have been shown 22 to increase Ruminococcaceae and Bifidobacteria while, at the same time, decreasing Clostridium species.
Saturated fat & your guts
Whilst coconut contains unusually high levels of saturated fat for a plant food, saturated fat is almost exclusively found in animal foods.
Studies suggest 23 that saturated fat activates systemic inflammation (by inducing pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α) and thus makes us much more vulnerable to systemic infections 24 and metabolic disorders 25 , such as type 2 diabetes and obesity.
Consuming high levels of saturated and trans fats – something increasingly common in the Western diet – increases the risk of cardiovascular disease and has been shown 6 26 to:
- increase
- Bilophila
- Faecalibacterium prausnitzii 27 , and
- Firmicutes
- decrease
Polyphenols
Polyphenols are secondary metabolites of plants and are generally involved in defence against ultraviolet radiation or aggression by pathogens 30 . These and other naturally occurring plant metabolites in plant foods have been shown to provide cardiovascular protection 6 as well as both anti-inflammatory and anti-pathogenic effects 31 .
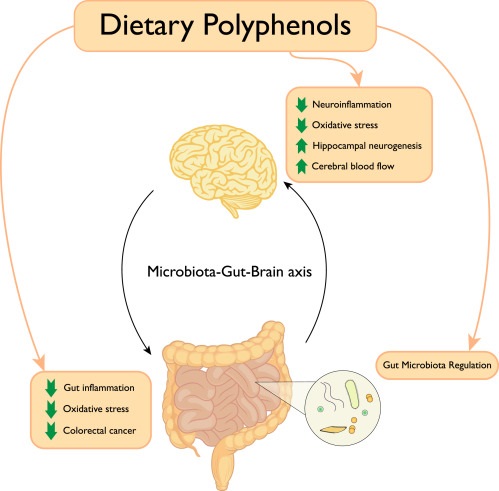
In plant foods, polyphenols increase:
- Bifidobacterium, and
- Lactobacillus
Whilst all plants have polyphenols, some of the most common polyphenol-rich foods include:
- fruits
- seeds
- vegetables
- tea
- cocoa products, and
- wine *
*N.B. The many negatives associated with alcohol consumption per se (for both gut health 32 and general health 33 ) suggest that the small quantities of polyphenols in wine are insufficient reason to drink the stuff.
Spice up you guts
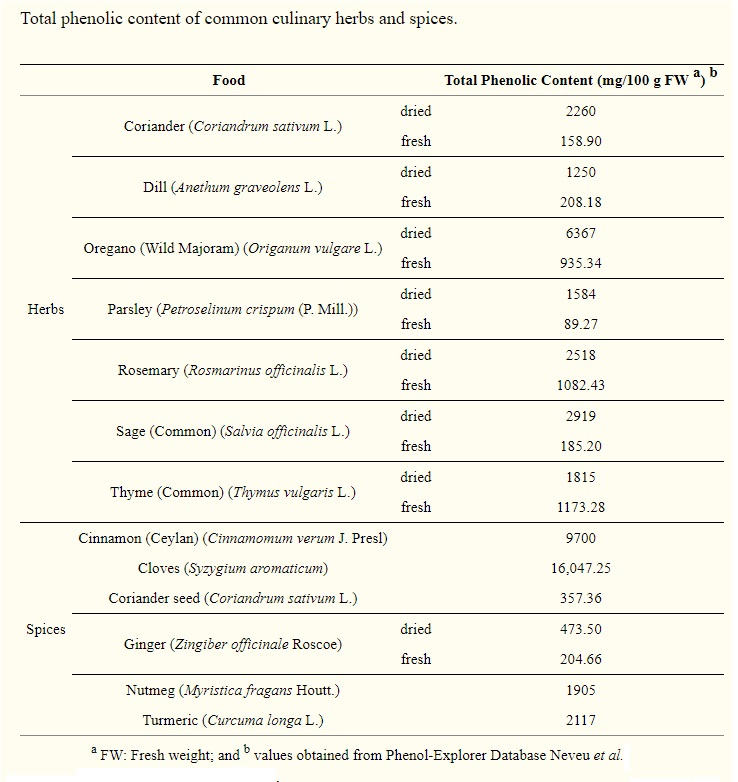
Spices and herbs are also very high in antioxidant polyphenols 34 , although the quantities that one can consume are, of course, limited.
And, of course, it’s widely known that tea contains high levels of polyphenols (including catechins, theaflavins, tannins, and flavonoids). Tea consumption increases Bifidobacterium and Lactobacillus–Enterococcus spp., something which appears 35 to result in increased SCFA production within human microbiota.
Postbiotics
Part One introduced this relatively new term. The postbiotics we’ll look at here are SCFAs, phytoestrogens, isothiocyanates, aryl-hydrocarbon receptor ligands, Coprostanol and secondary bile acids, trimethylamine N-oxide (TMAO), and vitamins.
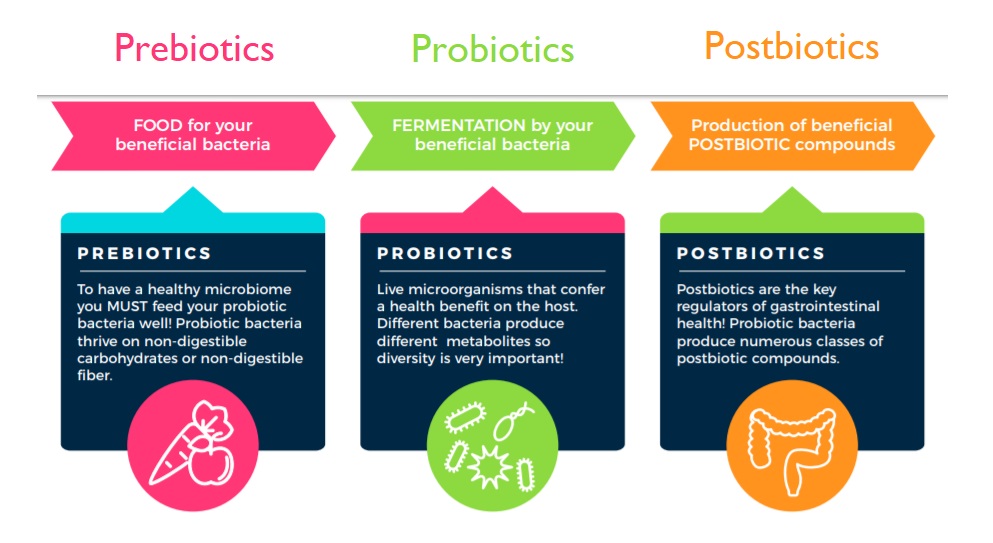
It’s important to understand the difference between prebiotics 36 /probiotics 37 on the one hand, and postbiotics, on the other. Basically, prebiotics (e.g. indigestible fibre) are put into the mouth and swallowed; probiotics are the microbes themselves which exist within the gut, but can also be consumed as dietary supplements; whilst postbiotics are the products of microbial activity within our guts.
Both prebiotics and postbiotics are, of course, vital for health. And to clarify once again: Probiotics are microbes that exist already in the GI tract, awaiting prebiotics (the substrate or source material). The products resulting from microbial activity are postbiotics – metabolites that research is showing are of fundamental importance for pretty much every functional system within the host (you and me) – from the gut-brain, gut-lung, and gut-liver axes, to immunoprotection 38 and mental health. 39 40 41
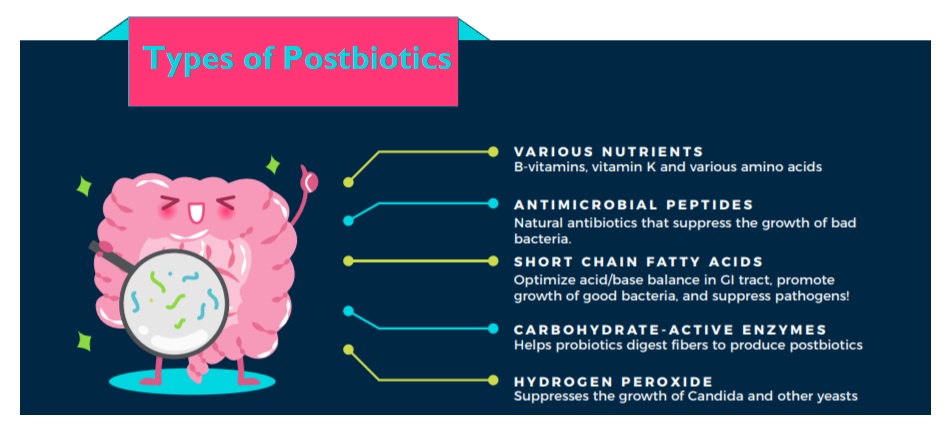
The various systems within our bodies are linked with each other via communication mechanisms that stem from the microbial products/metabolites (postbiotics) produced from the nutrients we ingest. As it happens, some products are diet-independent (for instance, lipopolysaccharides 42 , ribosomally synthesised and post-translationally modified peptides 43 etc.). We’ll set aside these diet-independent postbiotics, and look, instead, at the diet-dependent postbiotics mentioned above.
Location, location, location
The complexity of the human digestive system never fails to amaze. Not only do different foods encourage different microbes to produce different end products, but different locations along the intestinal tract result in different bioactive molecules being produced from the different prebiotics and nutrients 39 44 .
Diet & postbiotics
The type of food you eat is shown 45 to determines the range of postbiotic positive health effects that you enjoy, including:
- local anti-inflammatory and immunomodulatory 46
- antiobesogenic
- antihypertension
- anticholesterolemic
- antiproliferative 47 , and
- antioxidant
How postbiotics work
Postbiotic effects derive from a range of factors, including:
- modulation of gene expression
- metabolism
- intestinal function
- substrate composition
- microbiota composition
We’ll now look at the most well-known probiotics – i.e. SCFAs, phytoestrogens, isothiocyanates, aryl-hydrocarbon receptor ligands, Coprostanol and secondary bile acids, trimethylamine N-oxide (TMAO), and vitamins.
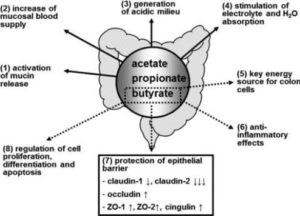
Short-Chain Fatty Acids (SCFAs)
The SCFAs acetate, propionate, and butyrate are mostly microbial metabolites of fermented fibre and other carbohydrates, although a tiny fraction does derive from proteins. Levels of these SCFAs significantly increases when a person begins to eat a plant-based diet 48 .
One of the roles of SCFAs is to act as a substrate for the maintenance of healthy colonic epithelium 49 . There is a correlation 50 between plant-based food consumption and improved epithelium health. Maintaining this intestinal barrier prevents endotoxemia 51 and subsequent inflammatory effects 52 53 .
Specific gut microbes are predisposed to produce SCFAs, and different bacteria produce different SCFAs, such as:
- acetate is produced by enteric bacteria 54 , such as:
- Akkermansia muciniphila
- Bifidobacterium spp.
- Prevotella spp., and
- Bacteroides spp.
- propionate is produced by:
- Bacteroides spp.
- butyrate is produced by:
- Coprococcus 55 , but mainly by
- Clostridium Cluster XIVa, IV, and XVI – species positively correlated with plant-food diets
These SCFAs would not be produced (or, at least, not produced in sufficiently high quantities to maintain optimal health) unless a largely or wholly plant-based diet were consumed.
SCFA protection
SCFAs (acetate, propionate and butyrate, in particular), protect against different types of disease, such as:
- type 2 diabetes 56
- inflammatory bowel disease 57
- immune diseases 58
- immunity against pathogens 55
- microglia 59 function and maturation/control of blood–brain barrier integrity 60
- thermogenesis 61 regulation 62
- preventing/treating NAFLD 63 and obesity 64
Propionate and gluconeogenesis
Gluconeogenesis (GNG) is a metabolic pathway that results in the generation of glucose from certain non-carbohydrate carbon substrates, such as protein and fat – and reverses the energy-production process of glycolysis. It provides the body’s cells with energy if carbohydrate stores are depleted. The SCFA, propionate acts as a gluconeogenic substrate in both the liver and intestine 55 . As well as helping to provide energy stores for the body, SCFAs are increasingly thought to play important roles as signalling molecules 65 .
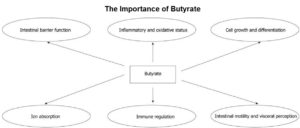
The beauty of butyrate
A previous blog 66 looked in some detail at butyrate. This SCFA, and the bacteria which produce it, are becoming increasingly accepted 55 as being highly beneficial to human health, including:
- acting as a major carbon source for colonocytes 67
- helping to regulate critical intestinal functions, such as 68 69 :
The foregoing barely scratches the surface of the vast range of functions and interactions of SCFAs; but the take-home message is that diets rich in fibre seem to provide huge benefits to both the intestine and overall health.
Phytoestrogens
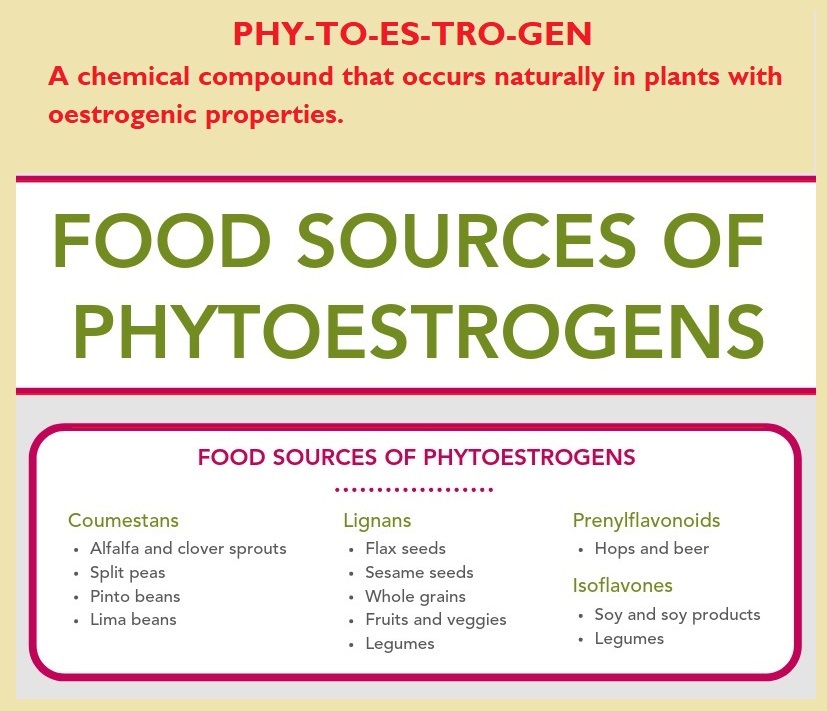
Phytoestrogens are plant-derived polyphenols that interact with oestrogen receptors with either agonist or antagonist actions 72 . They are found in various plant foods (e.g. seeds, grains, beans) and are concentrated in flax and soy – and, oddly enough, beer – the reason that beer-swilling men develop man boobs 73 .
Phytoestrogens appear 74 to have significant health-promoting properties. Research 75 76 77 has shown them to be:
- anticarcinogenic
- antidiabetic
- antiinflammatory
- antioxidant
- protective against cardiovascular disease
- antiobesogenic
- antidiabetic
- protective against osteoporosis and amyloid formation 78
Phytoestrogens have around a 1% bioavailability 79 , and so lots of them are able to get down to the gut. This is important because increasing evidence 80 81 suggests that the above positive health effects are only reached after bioactivation of the polyphenols by gut microbiota.
The players in polyphenol metabolism
As with most aspects of nutritional science, knowledge is limited about how many microbes are involved in polyphenol metabolism. However, the following are currently known 45 81 to be involved in the following process:
- converting polyphenols to equol 82 , urolithins 83 , and enterolignans 84 :
- Bifidobacterium
- Lactobacillus sp.
- Coriobacteriaceae
- Clostridium sp.
- Bacteroides, and
- Saccharomyces yeast
Coriobacteriaceae, Eubacterium and other species appear to be responsible for various other polyphenol transformations.
It works both ways
There’s a bidirectional relationship between gut microbiota and polyphenols 85 86 . That is, gut bacteria produce microbial metabolites (postbiotics) from polyphenols, and, in turn, these postbiotics act as prebiotics for various gut bacteria. The net result of this postbiotic production (especially the production of urolithins) encourages the growth of Lactobacillus and Bifidobacterium.
Isothiocyanates
 Sulforaphane, discussed in detail in a previous blog 87 in relation to the incredible health-giving power of broccoli, is perhaps the most extensively studied isothiocyanate – that is, a compound converted enzymatically from particular plant components called glucosinolates 88 . Isothiocyanates can be derived from cruciferous 89 or brassica 90 vegetables. The latter are rich sources of glucosinolate, the precursor of isothiocyanates.
Sulforaphane, discussed in detail in a previous blog 87 in relation to the incredible health-giving power of broccoli, is perhaps the most extensively studied isothiocyanate – that is, a compound converted enzymatically from particular plant components called glucosinolates 88 . Isothiocyanates can be derived from cruciferous 89 or brassica 90 vegetables. The latter are rich sources of glucosinolate, the precursor of isothiocyanates.
The following gut bacteria are largely responsible for facilitating the conversion of the glucosinolates in plant foods to the isothiocyanates our bodies need:
- Escherichia coli
- certain Bacteroides
- some Enterococcus
- Lactobacillus agilis
- certain Peptostreptococcus spp., and
- Bifidobacterium spp.
These bacteria secrete their own myrosinase enzyme 91 in order to metabolise the glucosinolates to isothiocyanates 92 .
Health benefits of isothiocyanates
Isothiocyanates are metabolites which are thought 93 94 to have a range of health-benefiting properties, including being:
- cytoprotective 95
- anticarcinogenic
- antioxidative
- antitumoural, and
- antiinflammatory
- detoxifying
Aryl-Hydrocarbon Receptor Ligands (AHRLs)
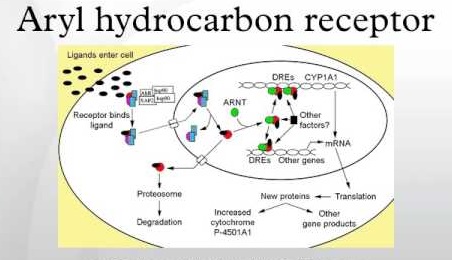 In terms of diet, intestinal aryl-hydrocarbon receptor 96 ligands 97 are mainly derived from eating plant food, especially cruciferous vegetables. Once again, gut bacteria are responsible for producing these AHRLs.
In terms of diet, intestinal aryl-hydrocarbon receptor 96 ligands 97 are mainly derived from eating plant food, especially cruciferous vegetables. Once again, gut bacteria are responsible for producing these AHRLs.
Using aryl-hydrocarbon receptors, these ligands are able to promote gut homeostasis and intestinal immune function 98 , as well as xenobiotic 99 detoxification and maintenance of energy metabolism, including lipid metabolism.
AHRLs and fat
A plant-based diet appears to be better at maintaining an appropriate level of AHRLs, while a high-fat diet appears to decrease the number of aryl-hydrocarbon receptor ligands. A decrease in either aryl-hydrocarbon receptors or in the associated ligands appears to compromise the healthy maintenance of intraepithelial lymphocytes 100 and the ability to control microbial load and composition. This can result in increased immune activation which can, in turn, cause epithelial damage 101 .
The result of this negative process can be gut permeability and inflammation. Both of these can promote the development of metabolic syndrome. Interestingly, some research suggests 98 102 that when metabolic syndrome is produced because of this process, the condition can be improved by supplementing the diet with a probiotic – namely, a strain of Lactobacillus.
Coprostanol and secondary bile acids
Dietary cholesterol is only found in animal-derived foods and, when consumed, it gets broken down in the gut by bacteria. The two resulting cholesterol metabolites (postbiotics) are coprostanol 103 and secondary bile acids.
Coprostanol is poorly absorbed by the human intestine after being isolated from cholesterol by several strains of gut bacteria. This is a good thing as far as cardiovascular disease risk is concerned, since it means that serum cholesterol in the host is reduced, with the coprostanol mostly being excreted in faeces rather than being absorbed back into the bloodstream 104 105 .
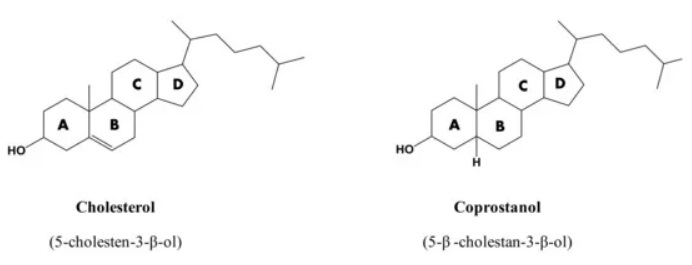
The situation is somewhat different when it comes to the other cholesterol postbiotic, secondary bile acids. One of the major uses of cholesterol is in the synthesis of bile acids in the liver. Bile acids are, of course, essential for the absorption of fat from the contents of the intestine; however, when the gut microbiota convert the bile acids synthesised from cholesterol into secondary bile acids 106 , they can be absorbed into the bloodstream and find their way into various tissues within the body. This is a problem.
Being hydrophobic 107 , these secondary bile acids are thought 108 capable of causing direct damage to cell membranes and inducing the generation of reactive oxygen species resulting in DNA damage, apoptosis 109 , and necrosis 110 . Additionally, it’s believed 104 44 that secondary bile acids are involved in maintaining the equilibrium of health and disease – being associated with inflammatory bowel disease, colon and liver cancer.
Trimethylamine N-Oxide (TMAO)
Trimethylamine N-oxide (TMAO) is a molecule generated from choline 111 , betaine 112 , and carnitine 113 via gut microbial metabolism. TMAO is associated with cardiovascular and neurological disorders. Carnitine and choline, precursors of TMAO, are mostly found in foods of animal origin (e.g. eggs, beef, pork), with lower amounts found in beans and fish 114 .
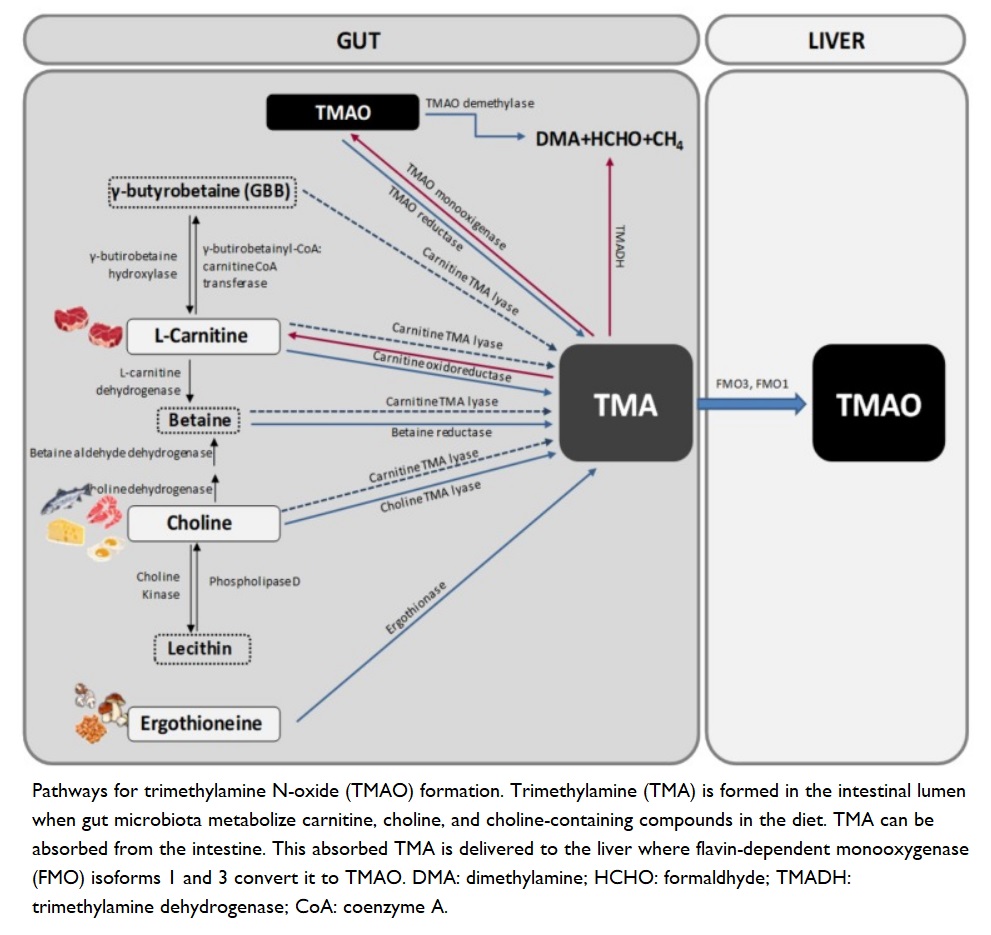
Diets containing animal proteins and fats (particularly red meat) tend to have decreased numbers of Bifidobacterium and increased numbers of L-Ruminococcus, Bacteroides, Alistipes, Ruminococcus, Clostridia, and Bilophila. Such diets are associated with elevated levels of TMAO 48 and, thereby, increased risk of cardiovascular disease and inflammatory bowel disease 23 6 .
The reason plant-eaters have a different gut microbiota composition to omnivores is that they have a reduced capacity to produce trimethylamine (TMA), the precursor to TMAO 115 . This reduced capacity appears to be due to both a reduction in the number of enzymes responsible for converting TMA to TMAO and to the general remodelling of gut microbiota that results from eating a plant-based diet.
Vitamins
We finally come to the gut microbiota which are essential for producing and maintaining adequate vitamin levels within our bodies.
Not a lot of people know this, but our gut microbes produce or process several vital vitamins 93 :
- menaquinone (vitamin K2)
- thiamine (vitamin B1)
- riboflavin (vitamin B2)
- niacin (vitamin B3)
- pantothenic acid (vitamin B5)
- pyridoxine (vitamin B6)
- biotin (vitamin B7)
- folate (vitamin B9)
- cobalamin (vitamin B12*)
* N.B. vitamin B12, while being produced by gut bacteria, is not absorbed back in to the body. This means that vitamin B12 needs to be taken as a supplement by vegans and, arguably, by most other people irrespective of their dietary choices. This is discussed in great detail in previous blogs 116 117 118 119 .
Different bacteria possess specific biosynthetic properties for different vitamins, such as:
- Bifidobacterium – vitamins K, B1, B7, B9, and B12
- Bacillus subtilis and Escherichia coli – riboflavin 120
- Lactobacillus – B12, and other B vitamins 121
The latter is by no means a comprehensive analysis of the relationship between intestinal bacteria and vitamin production/processing; but it does provide a brief insight into one more essential role played by the microbes that live within us.
Final thoughts
It’s thought that, on average, around 25% of the plasma metabolites resulting from gut microbial activity are different between omnivores and vegans, with current research consistently indicating that diet is the essential factor for the composition and health of human gut microbiota. In turn, this is vital for metabolising the nutrients we consume into postbiotics that our bodies need.
All known research continues to suggest that a plant-based diet may be the most effective way of promoting a diverse ecosystem of beneficial microbes that can support overall health. Nutrition is a complex field, with inter-individual differences abounding. This means that further research is necessary if we are every going to be able to fully characterise the interactions between microbiome, diet and health.
But, in the meantime, it looks like you’d be doing your overall health a huge favour if you choose to…

References & Notes
- SCFAs – short-chain fatty acids [↩]
- Eat Enough Food & You Eat Enough Protein [↩]
- Animal Protein & Your Kidneys [↩]
- The Problem with Protein [↩]
- THE PROTEIN COMBINING MYTH – A RAT’S TALE ? [↩]
- Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, et al. . Influence of diet on the gut microbiome and implications for human health. J Transl Med. (2017) 15:73. 10.1186/s12967-017-1175-y. [↩] [↩] [↩] [↩] [↩] [↩]
- Alistipes: Wikipedia. [↩]
- Bilophila wadsworthia: Wikipedia. [↩]
- Bacteroides: Wikipedia. [↩]
- Clostridia: Wikipedia. [↩]
- How to Reduce Carcinogenic Bile Acid Production: Video by Dr Michael Greger. [↩]
- Hentges DJ, Maier BR, Burton GC, Flynn MA, Tsutakawa RK. Effect of a high-beef diet on the fecal bacterial flora of humans. Cancer Res. (1977) 37:568–71. [↩]
- Roseburia: Wikipedia. [↩]
- Eubacterium: Wikipedia. [↩]
- Ruminococcus: Wikipedia. [↩]
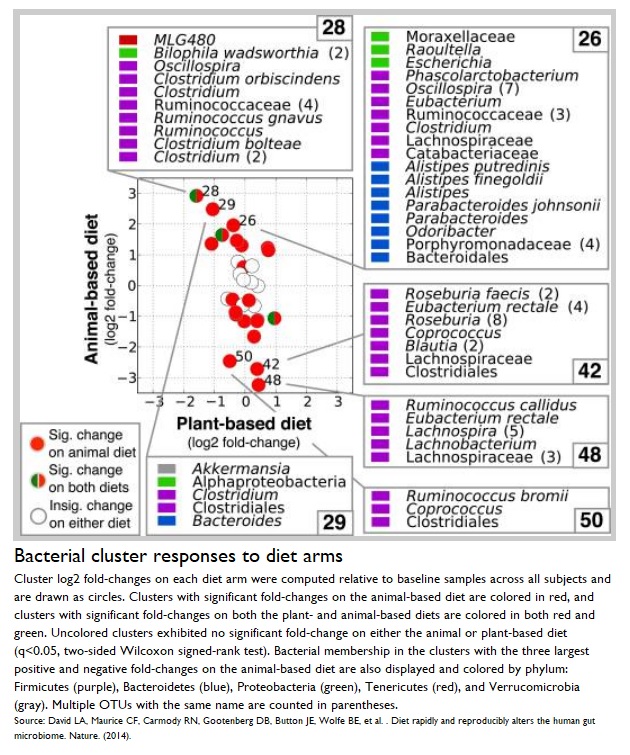
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. . Diet rapidly and reproducibly alters the human gut microbiome. Nature. (2014) 505:559–63. 10.1038/nature12820. [↩]
- Zer0-sum game: Wikipedia. [↩]
- Sheflin AM, Melby CL, Carbonero F, Weir TL. Linking dietary patterns with gut microbial composition and function. Gut Microbes. (2016) 8:113–29. 10.1080/19490976.2016.1270809. [↩] [↩]
- Front Microbiol. 2016; 7: 925. Bifidobacteria and Their Role as Members of the Human Gut Microbiota
Amy O’Callaghan and Douwe van Sinderen. [↩] - Why the Bacteroidetes:Firmicutes ratio matters [↩]
- Non-Fish Sources of Omega-3 [↩]
- Bamberger C, Rossmeier A, Lechner K, Wu L, Waldmann E, Fischer S, et al. . A walnut-enriched diet affects gut microbiome in healthy caucasian subjects: a randomized, controlled trial. Nutrients. (2018) 10. 10.3390/nu10020244 [↩]
- Lee YK. Effects of diet on gut microbiota profile and the implications for health and disease. Biosci Microbiota Food Health. (2013) 32:1–12. 10.12938/bmfh.32.1 [↩] [↩]
- Deitch EA. 1990. Bacterial translocation of the gut flora. J Trauma 30:(Suppl): S184–S189 [↩]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. 2007a. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772 [↩]
- Coelho OGL, Cândido FG, Alfenas RCG. Dietary fat and gut microbiota: mechanisms involved in obesity control. Crit Rev Food Sci Nutr. (2018) 2018:1–9. 10.1080/10408398.2018.1481821 [↩]
- Faecalibacterium: Wikipedia. [↩]
- ssp. is short for sub-species [↩]
- spp. is short for species [↩]
- Polyphenols: nutritionfacts.org topic. [↩]
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. (2009) 2:270–8. 10.4161/oxim.2.5.9498 [↩]
- Alcohol – Bad News for Good Bacteria [↩]
- No Amount of Alcohol Consumption is Safe [↩]
- Antioxidants in a Pinch. Michael Greger M.D. FACLM January 18th, 2012 Volume 7. [↩]
- Sun H, Chen Y, Cheng M, Zhang X, Zheng X, Zhang Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J Food Sci Technol. (2018) 55:399–407. 10.1007/s13197-017-2951-7 [↩]
- Prebiotics are compounds in food – largely indigestible fibre – that induce the growth or activity of beneficial microorganisms such as bacteria and fungi. The most common example is in the gastrointestinal tract, where prebiotics can alter the composition of organisms in the gut microbiome. [↩]
- Probiotics are live microorganisms intended to provide health benefits when consumed, generally by improving or restoring the gut flora. [↩]
- The immune system protects against invasion by foreign substance – and antigen – that can cause the body to produce antibodies. [↩]
- Klemashevich C, Wu C, Howsmon D, Alaniz RC, Lee K, Jayaraman A. Rational identification of diet-derived postbiotics for improving intestinal microbiota function. Curr Opin Biotechnol. (2014) 26:85–90. 10.1016/j.copbio.2013.10.006 [↩] [↩]
- Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. (2011) 23:187–92. 10.1111/j.1365-2982.2010.01664.x [↩]
- Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Cartenì M, et al. . Gut–liver axis: The impact of gut microbiota on non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. (2012) 22:471–6. 10.1016/j.numecd.2012.02.007 [↩]
- Lipopolysaccharides (LPS), also known as lipoglycans and endotoxins, are large molecules consisting of a lipid and a polysaccharide composed of O-antigen, outer core and inner core joined by a covalent bond; they are found in the outer membrane of Gram-negative bacteria. [↩]
- Ribosomally synthesised and post-translationally modified peptides (RiPPs), also known as ribosomal natural products, are a diverse class of natural products of ribosomal origin. [↩]
- Donia MS, Fischbach MA. Human microbiota. Small molecules from the human microbiota. Science. (2015) 349:1254766. 10.1126/science.1254766 [↩] [↩]
- Aguilar-Toalá JE, Garcia-Varela R, Garcia HS, et al. Postbiotics: An evolving term within the functional foods field. Trends Food Sci Technol. (2018) 75:105–14. 10.1016/j.tifs.2018.03.009 [↩] [↩]
- Immunomoduation is modulation (normal and healthy regulatory adjustment) of the immune system. [↩]
- Antiproliferative – tending to inhibit cell growth, specifically of tumour cells. [↩]
- De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. . High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. (2016) 65:1812–21. 10.1136/gutjnl-2015-309957 [↩] [↩]
- The intestinal epithelium is the single cell layer that form the luminal surface (lining) of both the small and large intestine (colon) of the gastrointestinal tract. [↩]
- Zhang C, Björkman A, Cai K, Liu G, Wang C, Li Y, et al. . Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front Immunol. (2018) 9:908. 10.3389/fimmu.2018.00908 [↩]
- Endotoxins are part of the outer membrane of the cell wall of Gram-negative bacteria. Endotoxemia is the presence of endotoxins in the blood. If these are derived from gram-negative rod-shaped bacteria, they may cause haemorrhages, necrosis of the kidneys, shock, etc. [↩]
- Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. (2016) 7:189–200. 10.1080/19490976.2015.1134082 [↩]
- Cani PD, Bibiloni R, Knauf C, et al. . Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. (2008) 57:1470–81. 10.2337/db07-1403 [↩]
- Enteric bacteria are intestinal bacteria which are always present and are usually harmless. [↩]
- Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. (2016) 165:1332–45. 10.1016/j.cell.2016.05.041 [↩] [↩] [↩] [↩]
- A high fiber diet may promote specific short-chain fatty acid producers to achieve better metabolic control in type 2 diabetes. 26 MAR 2018. Andreu Prados. [↩]
- Front Immunol. 2019 Mar 11;10:277. doi: 10.3389/fimmu.2019.00277. eCollection 2019. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Parada Venegas D et al. [↩]
- PLoS One. 2017; 12(2): e0173032. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. Miho Mizuno et al [↩]
- Microglia are a type of neuroglia (glial cell) located throughout the brain and spinal cord. Microglia account for 10–15% of all cells found within the brain. As the resident macrophage cells, they act as the first and main form of active immune defense in the central nervous system (CNS). [↩]
- Erny D, Hrabě de Angelis AL, Prinz M. Communicating systems in the body: how microbiota and microglia cooperate. Immunology. (2017) 150:7–15. 10.1111/imm.12645 [↩]
- Thermogenesis is a metabolic process during which your body burns calories to produce heat. Several factors induce thermogenesis in your body including exercise, diet and environmental temperature. Thermogenesis can promote weight loss because it increases your body’s calorie burn. [↩]
- Reynés B, Palou M, Rodríguez AM, Palou A. Regulation of adaptive thermogenesis and browning by prebiotics and postbiotics. Front Physiol. (2019) 9:1908. 10.3389/fphys.2018.01908 [↩]
- Non-alcoholic fatty liver disease [↩]
- Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. (2019). 10.1038/s41574-019-0156-z [↩]
- Front. Endocrinol., 05 June 2014. The SCFA receptor GPR43 and energy metabolism. Ikuo Kimura et al [↩]
- Butyrate – Why Dietary Fibre is So Important [↩]
- Colonocytes is a name used for the epithelial cells of the colon. [↩]
- Velázquez OC, Lederer HM, Rombeau JL. Butyrate and the colonocyte. Production, absorption, metabolism, and therapeutic implications. Adv Exp Med Biol. (1997) 427:123–34. 10.1007/978-1-4615-5967-2_14 [↩]
- Borycka-Kiciak K, Banasiewicz T, Rydzewska G. Butyric acid – a well-known molecule revisited. Przeglad Gastroenterol. (2017) 12:83–9. 10.5114/pg.2017.68342 [↩]
- A healthy mucosa contains a steep oxygen gradient along the length of the intestine and from the lumen to the serosa – the mucosa surrounds the lumen, or open space within the digestive tube. [↩]
- Am J Physiol Cell Physiol. 2015 Sep 15; 309(6): C350–C360. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Leon Zheng, Caleb J. Kelly, and Sean P. Colgan. [↩]
- An agonist is a chemical that binds to a receptor and activates the receptor to produce a biological response. An agonist causes an action, while an antagonist blocks the action of the agonist. [↩]
- The Most Potent Phytoestrogen Is In Beer. Video by Michael Greger M.D. FACLM July 27th, 2016 Volume 31 [↩]
- Phytoestrogens: Topic on nutritionfacts.org. [↩]
- Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. (2016) 5:e47, 1–15. 10.1017/jns.2016.41 [↩]
- Ono K, Li L, Takamura Y, Yoshiike Y, Zhu L, Han F, et al. . Phenolic compounds prevent amyloid β-protein oligomerization and synaptic dysfunction by site-specific binding. J Biol Chem. (2012) 287:14631–43. 10.1074/jbc.M111.325456 [↩]
- Hossen MS, Ali MY, Jahurul MHA, Abdel-Daim MM, Gan SH, Khalil MI. Beneficial roles of honey polyphenols against some human degenerative diseases: a review. Pharmacol Rep. (2017) 69:1194–205. 10.1016/j.pharep.2017.07.002 [↩]
- In general, amyloid formation, or amyloidosis, is caused by the buildup of an abnormal protein called amyloid. Amyloid is produced in bone marrow and can be deposited in any tissue or organ. The antibodies that get produced are deposited in tissues as amyloid, interfering with normal function. [↩]
- Ercolini D, Fogliano V. Food design to feed the human gut microbiota. J Agric Food Chem. (2018) 66:3754–8. 10.1021/acs.jafc.8b00456 [↩]
- Landete JM, Arqués J, Medina M, Gaya P, de Las Rivas B, Muñoz R. Bioactivation of phytoestrogens: intestinal bacteria and health. Crit Rev Food Sci Nutr. (2016) 56:1826–43. 10.1080/10408398.2013.789823 [↩]
- Tomás-Barberán FA, González-Sarrías A, García-Villalba R, Núñez-Sánchez MA, Selma MV, García-Conesa MT, et al. . Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol Nutr Food Res. (2017) 61:1. 10.1002/mnfr.201500901 [↩] [↩]
- Equol is an isoflavandiol oestrogen metabolised from daidzein, a type of isoflavone found in soybeans and other plant sources, by bacterial flora in the intestines. While endogenous oestrogenic hormones such as estradiol are steroids, equol is a nonsteroidal oestrogen. [↩]
- Urolithins are the major metabolites of polyphenols in the gut, being produced by bacteria when breaking down foods such as pomegranates, nuts and berries. Urolithins have been studied for their antioxidant, antiinflammatory, antioestrogenic properties and their anticancer effects. [↩]
- Enterolignans are one of a wide range of lignans found in plants. Plant lignans can be converted by various intestinal bacteria to enterolignans, enterodiol and enterolactone. Enterolignans have a variety of biologic activities, including tissue-specific oestrogen receptor activation, and antiinflammatory and apoptotic effects, that may influence disease risk in humans. [↩]
- Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. (2013) 24:1415–22. 10.1016/j.jnutbio.2013.05.001 [↩]
- Ozdal T, Sela DA, Xiao J, Boyacioglu D, Chen F, Capanoglu E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients. (2016) 8:2. 10.3390/nu8020078 [↩]
- Broccoli & Sulforaphane vs Cancer [↩]
- Glucosinolates are natural components of many pungent plants such as mustard, cabbage, and horseradish. The pungency of those plants is due to mustard oils produced from glucosinolates when the plant material is chewed, cut, or otherwise damaged. [↩]
- Cruciferous vegetables include plants such as bok choi, broccoli, Brussels sprouts, cabbage, cauliflower, horseradish, kale, kohlrabi, mustard, radish, rutabaga, turnip, and watercress. [↩]
- The brassica (or Brassicaceae) family can also be called the Cruciferae family. They are a taxonomic family which includes many genera and cultivars. The brassica family takes its alternative name (Cruciferae, New Latin for “cross-bearing”) from the shape of their flowers, whose four petals resemble a cross. [↩]
- Myrosinase enzymes, such as thioglucoside glucohydrolase, sinigrinase, and sinigrase, is a family of enzymes involved in plant defence against herbivores. Its known biological function is to catalyse the hydrolysis of a class of compounds called glucosinolates. [↩]
- Tian S, Liu X, Lei P, Zhang X, Shan Y. Microbiota: a mediator to transform glucosinolate precursors in cruciferous vegetables to the active isothiocyanates. J Sci Food Agric. (2018) 98:1255–60. 10.1002/jsfa.8654 [↩]
- Derrien M, Veiga P. Rethinking diet to aid human-microbe symbiosis. Trends Microbiol. 2017. [↩] [↩]
- Recent Pat Endocr Metab Immune Drug Discov. 2013 Sep;7(3):213-25. The anti-oxidant properties of isothiocyanates: a review. de Figueiredo SM, Filho SA, Nogueira-Machado JA, Caligiorne RB. [↩]
- Cytoprotection is a process by which chemical compounds provide protection to cells against harmful agents. For example, a gastric cytoprotectant is any medication that combats ulcers not by reducing gastric acid but by increasing mucosal protection. [↩]
- The aryl hydrocarbon receptor is a protein that in humans is encoded by the AHR gene. The aryl hydrocarbon receptor is a transcription factor within cells to regulate gene expression. [↩]
- In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. For instance, in protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. [↩]
- Natividad JM, Lamas B, Pham HP, Michel ML, Rainteau D, Bridonneau C, et al. . Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat Commun. (2018) 9:2802. 10.1038/s41467-018-05249-7 [↩] [↩]
- A xenobiotic is a chemical substance found within an organism that is not naturally produced or expected to be present within the organism. It can also cover substances that are present in much higher concentrations than are usual. [↩]
- Intraepithelial lymphocytes (IEL) are lymphocytes found in the epithelial layer of mammalian mucosal linings, such as the gastrointestinal (GI) tract and reproductive tract. [↩]
- Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, et al. . Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. (2011) 147:629–40. 10.1016/j.cell.2011.09.025 [↩]
- Immunol Cell Biol. 2011 Oct;89(7):817-22. doi: 10.1038/icb.2010.165. Epub 2011 Feb 15. Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Takamura T, et al. [↩]
- Coprostanol is the major sterol in human faeces, and has been routinely studied as a marker of (modern) sewage pollution in marine and lacustrine sediments. This has led to the search for coprostanol in archaeological soils, in order to detect the presence of faecal material. [↩]
- Gérard P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens. (2013) 3:14–24. 10.3390/pathogens3010014 [↩] [↩]
- Horáčková Š, Plocková M, Demnerová K. Importance of microbial defence systems to bile salts and mechanisms of serum cholesterol reduction. Biotechnol Adv. (2018) 36:682–90. 10.1016/j.biotechadv.2017.12.005 [↩]
- Secondary bile acids (or salts) result from the secretion of primary bile acids into the lumen of the intestine. Bacteria partially dehydroxylate them and remove the glycine and taurine groups. The primary bile acids, cholic acid and chenodeoxycholic acid, are converted into the secondary bile acids, deoxycholic acid and lithocholic acid, respectively. [↩]
- Hydrophobic molecules and surfaces repel water. Hydrophobic liquids, such as oil, will separate from water. Hydrophobic molecules are usually nonpolar, meaning the atoms that make the molecule do not produce a static electric field. [↩]
- Bile Acids – an overview. ScienceDirect Topics. https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular…/bile-acids [↩]
- Apoptosis is the death of cells which occurs as a normal and controlled part of an organism’s growth or development. [↩]
- Necrosis is the death of most or all of the cells in an organ or tissue due to disease, injury, or failure of the blood supply. [↩]
- Choline is particularly rich in eggs, liver, and peanuts, and is also found in meat, poultry, fish, dairy foods, pasta, and rice. [↩]
- Betaine is a metabolite of choline and is a nonessential nutrient found in numerous food sources, including sugar beets, wheat bran, rye grain, bulgar grain, spinach, quinoa, brown rice, sweet potato, turkey breast, beef, veal and some seafood, such as shrimp. [↩]
- Red meat contains the highest level of carnitine. It is also found in smaller amounts in chicken, milk and dairy products, fish, beans, and avocado. Vegans tend to get less carnitine from foods, and their bodies usually produce enough naturally without requiring any from their diet. [↩]
- Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M. Implication of trimethylamine N-Oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. (2018) 10:10 10.3390/nu10101398 [↩]
- Obeid R, Awwad HM, Keller M, Geisel J. Trimethylamine-N-oxide and its biological variations in vegetarians. Eur J Nutr. (2017) 56:2599–609. 10.1007/s00394-016-1295-9 [↩]
- A General Plant-Eater’s Mistake #2? [↩]
- Vegan Society Veg-1: Does It Contain Enough B12? [↩]
- B12 Supplements Are Efficient But Caution With Folic Acid [↩]
- Vitamin B12 Status in Spanish Veggies & Vegans [↩]
- De Filippis F, Pellegrini N, Laghi L, Gobbetti M, Ercolini D. Unusual sub-genus associations of faecal Prevotella and Bacteroides with specific dietary patterns. Microbiome. (2016) 4. 10.1186/s40168-016-0202-1 [↩]
- LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. (2013) 24:160–8. 10.1016/j.copbio.2012.08.005 [↩]


 The gooey mass travels through the oesophagus and into the rumen. The rumen acts as a fermentation tank since huge quantities of bacteria
The gooey mass travels through the oesophagus and into the rumen. The rumen acts as a fermentation tank since huge quantities of bacteria  As the bacteria digest the plant matter, they produce by-products including VFAs (volatile fatty acids)
As the bacteria digest the plant matter, they produce by-products including VFAs (volatile fatty acids)  Grasses and forbs
Grasses and forbs  Well, not strictly true – but they do often swallow metal objects (such as nails or wire) when foraging, and farmers sometimes make cows swallow magnets. But why?
Well, not strictly true – but they do often swallow metal objects (such as nails or wire) when foraging, and farmers sometimes make cows swallow magnets. But why?