What happens “down below” in our intestines is of such vital importance to our health that I’ve previously covered aspects of this subject in relation to a variety of specific subjects, such as alcohol 1 , obstructive sleep apnea 2 , depression 3 , physical activity 4 , and multiple sclerosis 5 . However, a new review 6 just published puts more meat on the bone – or more fruit on the tree!
The review looks at how the food we put in our mouths determines the type and quantity of our gut flora (also known as gut bacteria, microbes, microbiota or microbiome) and, in turn, how these dietary choices affect the direct and indirect actions performed by these vital microscopic inhabitants of our bodies.
This is Part One of a two part blog on this study. Here, we’ll look at the types and activities of microbes found in the intestinal microbiome of those eating plant- and meat-based diets, finally focusing on the microbial effects of consuming the macronutrient, carbohydrate.
In Part Two, we’ll look at the microbial effects of the other two macronutrients (protein and fat), polyphenols, and the influence on human health of microbiome postbiotics7 derived from such things as vitamins and TMAO.
Blog Contents
Definition of terms
“Microbiota”and “microbiome” tend to be used interchangeably; however, “microbiota” refers to the total of all microbial taxa associated with humans (including bacteria, viruses, fungi, protozoa and archaea), while “microbiome” refers to the complete catalogue of microbes plus all their genes.
Each human’s gut microbiota is estimated 8 to consist of over 3 trillion microbes – although I don’t know if anyone has actually sat down and counted them…
The human gut microbiome consists of around 3.3 million non-redundant 9 microbial genes. Amazingly, this is much more than our human genome, which only contains around 21,000 genes. So there are around 150 distinct microbial genes in our guts for every one of our human genes 10 .
Omnivore vs plant-based microbiota
Previous blogs 11 12 have looked at the evidence for significant differences between the microbiota of meat eaters and plant eaters. Plant-based diets appear to promote the development of more diverse and stable microbial systems, with significantly more Bacteroidetes-related operational taxonomic units compared to omnivores.
Important components of a plant-based diet include:
Fibre (non-digestible carbohydrates that are only found in plants) increase lactic acid bacteria (e.g. Roseburia, Ruminococcus, and E. rectale) while reducing certain pathogenic bacteria (e.g. Clostridium and Enterococcus species). Lactic acid bacteria can help improve lactose digestion, prevent and treat diarrhoea, and act on the immune system, helping the body to resist and fight infection.
SCFAs (short-chain fatty acids) have a plethora of health benefits. A diet high in fibre encourages species that ferment fibre into metabolites in the form of SCFAs (such as acetate, propionate and buyrate). We previously looked in detail at butyrate 13 and saw how such bacteria-produced SCFAs can improve immunity against pathogens, increase the integrity of the blood–brain barrier, provide energy substrates, and regulate critical intestinal functions.
And it’s not just SCFAs that mark the difference between plant- and meat-based diets – for instance, the former contains phytoestrogens, and isothiocyanates such as sulforaphane 14 , whilst the latter contains TMAO 15 and secondary bile acids 16 . These and other basic constituents of each diet will determine the type of gut bacteria that can survive within your body. We’ll look at the latter in more detail in Part Two.
Polyphenols (again, only found in plants) increase bacteria species which can provide anti-pathogenic/anti-inflammatory properties as well as cardiovascular protection (e.g. Bifidobacterium and Lactobacillus).
It’s no surprise, then, that a diet which is high in the foregoing will likely result in a diverse ecosystem of beneficial bacteria that supports the health of the host – that is, you and me.
Diversity & distribution matter
Plant-based diets also appear to promote good health by developing a richer, more diverse gut microbial system, and/or by producing an even distribution of a variety of species 17 18 .
Microbiome is a separate “organ”
The range of functions of the human gut microbiome is so wide-ranging (immunity, gastrointestinal, brain, cardiovascular systems, cellular and genetic activity) that it’s increasingly common for researchers to regard it as a distinct “organ” within the human body.
Three basic bacterial enterotypes
Several studies 19 20 have suggested that there are three basic bacterial enterotypes 21 :
- genus Prevotella (largely anti-inflammatory and protective)
- genus Bacteroides (more pro-inflammatory and possible associations with heightened risk of metabolic syndrome and other pathological conditions)
- genus Ruminococcus (whose biological significance is still largely unclear)
The effects of imbalanced gut microbiota
Imbalanced gut microbiota has been linked 22 23 24 25 with a surprisingly large number of conditions, including the following:
- acid reflux 26
- peptic ulcers 27
- irritable bowel syndrome 28
- non-alcoholic liver disease 29
- inflammatory bowel disease 30
- obesity 31
- atherosclerosis 32
- type 2 diabetes 33
- cancer
- Alzheimer’s 34
- Parkinson’s 35
- motor neurone disease 36
- lateral sclerosis 37
- autism spectrum disorder 38 39
- atopy40 41
Microbiota and personalised nutrition
Because of the amount of evidence being accumulated in support of the role of our gut microbiota in acting as a mediator of dietary impact on the host metabolic status, an increasing amount of research is being focused on establishing causal relationships in individual people between the food they eat, what it does to their gut microbiota and the consequent effects on their overall health. It’s anticipated that this will allow for the development of therapeutic interventions such as personalised nutrition (18).
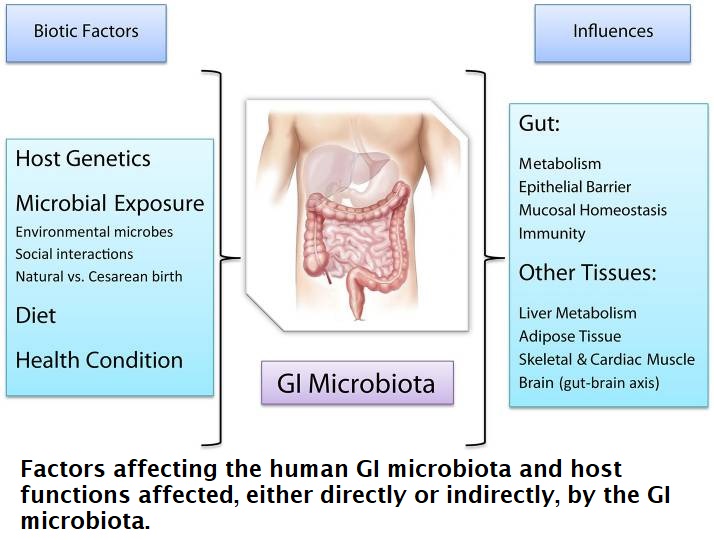
One such study concludes: “Convincing accumulating evidence shows that the human gut microbiota contributes to many aspects of human health via molecular pathways that we only begin to understand. The GI microbiota entertains deep mutualistic relationships and co-evolves with the human host, albeit at a much faster rate and demonstrates deep ecological links with the host which are being studied at the interface between Biology, Ecology, and Medicine. Experimental probing of the deep and reciprocal ties characterizing the microbiota-host relationship constitutes a formidable challenge, yet holds the promise to shed light on unknown aspects of human and microbial physiology and novel therapeutic possibilities in the manipulation of microbiota composition via antibiotics, probiotics, and microbial transplantation.” 42
Microbiota and postbiotics
The term “postbiotics” is relatively new. It refers to the vast range of compounds (metabolites) produced by the metabolic activity of our gut bacteria depending, to a large extent, on what nutrients they receive from the diet we eat. These probiotic-produced postbiotic compounds play vital roles in the regulation, not only of the host’s health, but also in the maintenance of a healthy gut microbiome.
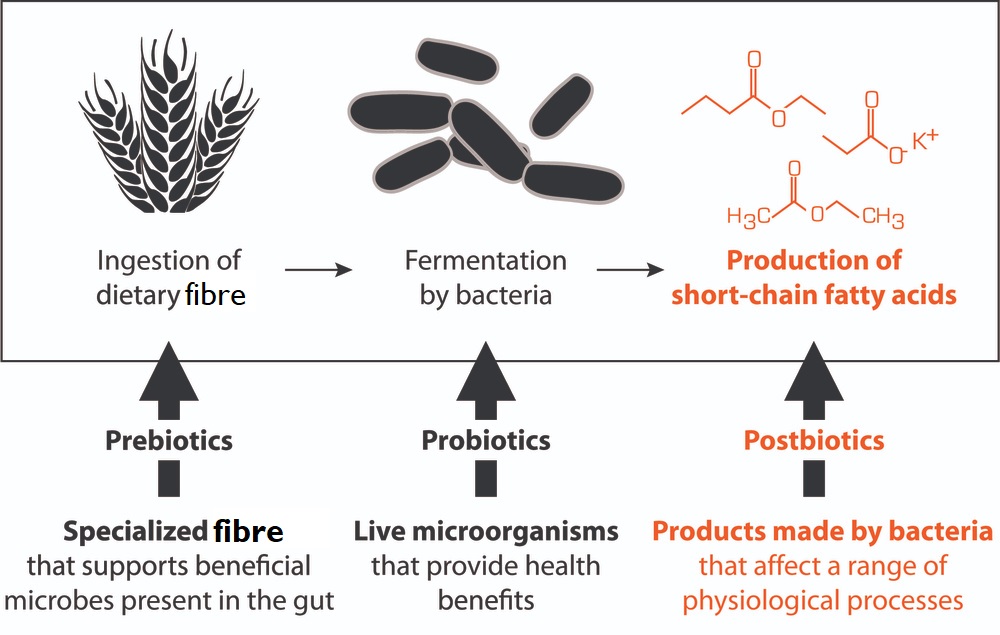
The postbiotics produced by probiotic bacteria appear to be responsible for many of the beneficial effects claimed for probiotics.
N.B. In most cases, it can be argued that a healthy and balanced WFPB diet will provide all the natural probiotics you need without having to resort to commercially-produced stuff with dubious effectiveness or, indeed, safety. 43 44 Whole plants are covered with bacteria that have been interacting effectively with our bodies for millions of years; and the postbiotics produced by microbes inside vegetarians/vegans have been shown 45 to be particularly effective in reducing various risk factors for chronic inflammation and chronic degenerative diseases.
What determines gut microbiota composition?
Variations in microbiota composition from one person to another are likely to be a combination of the following:
- different directly-consumed foodborne bacteria 46
- differences in the substrates 47
- variations in transit time through the GI tract 48
- pH level 49
- host secretion influenced by dietary patterns 50
- regulation of gene expression of the host and/or his/her microbiota 51 45
Gut Microbiota – Why diversity matters
Microbial diversity associated with plant-based diets – particularly seen in research on long-term fruit, vegetable and whole grain intake 52 53 – appears to have important associations with many health indicators, including:
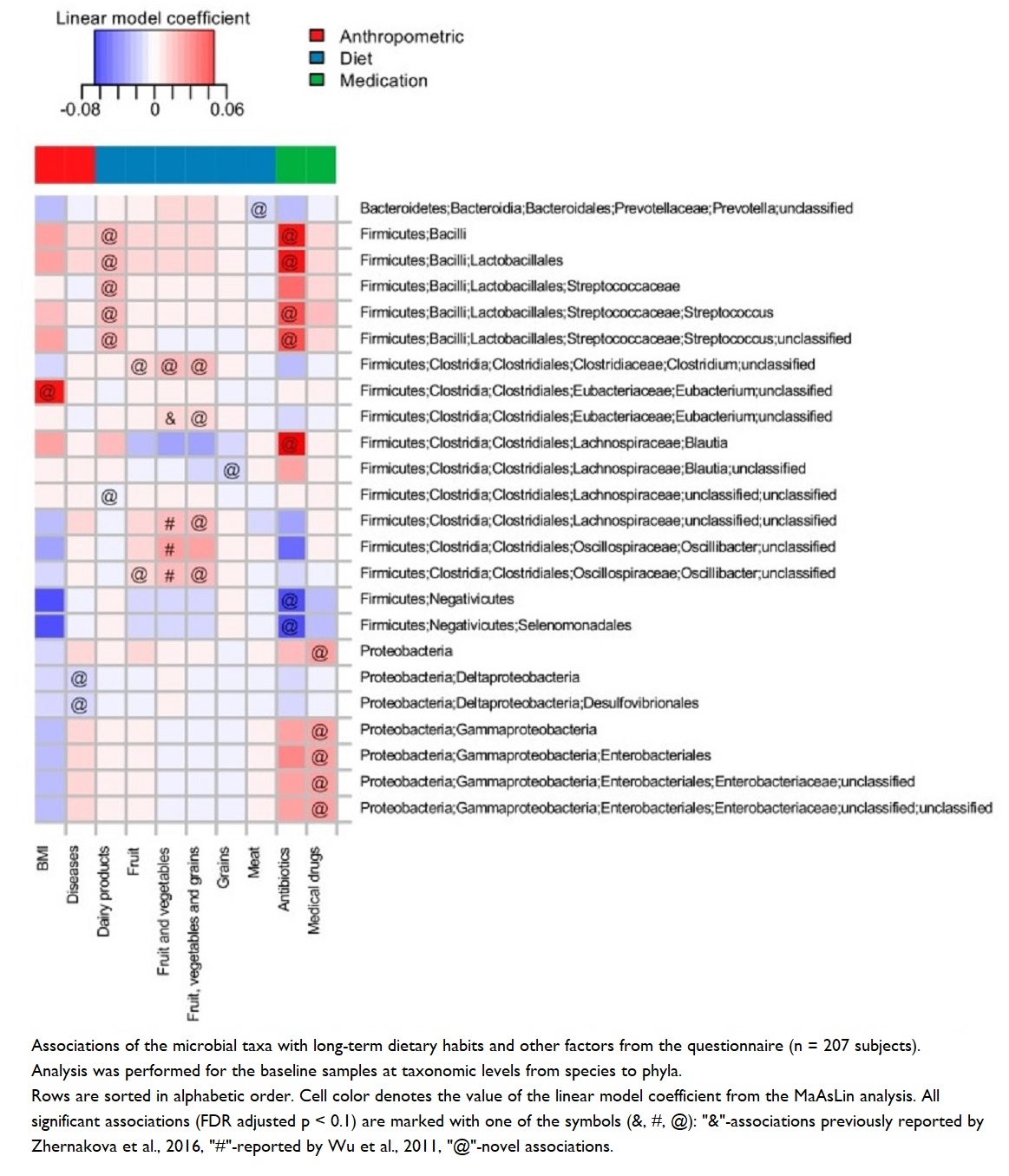
It takes time to achieve optimal gut health
Dietary changes are shown 56 to have relatively rapid impact (within a week) on microbial composition and, hence, on the effects of their metabolites. However, it’s important to note that these effects can be modest and reversible if the individual reverts to their prior dietary habits 51 .
One study showed 57 that changes in microbiota and associated immune parameters after just 3 months on a vegetarian (not a WFPB or vegan) diet can be pretty significant; but these changes pale into comparison when compared with the degree of change that occurs with a long-term plant-based diet.
Microbial “stress” and high-fibre diets
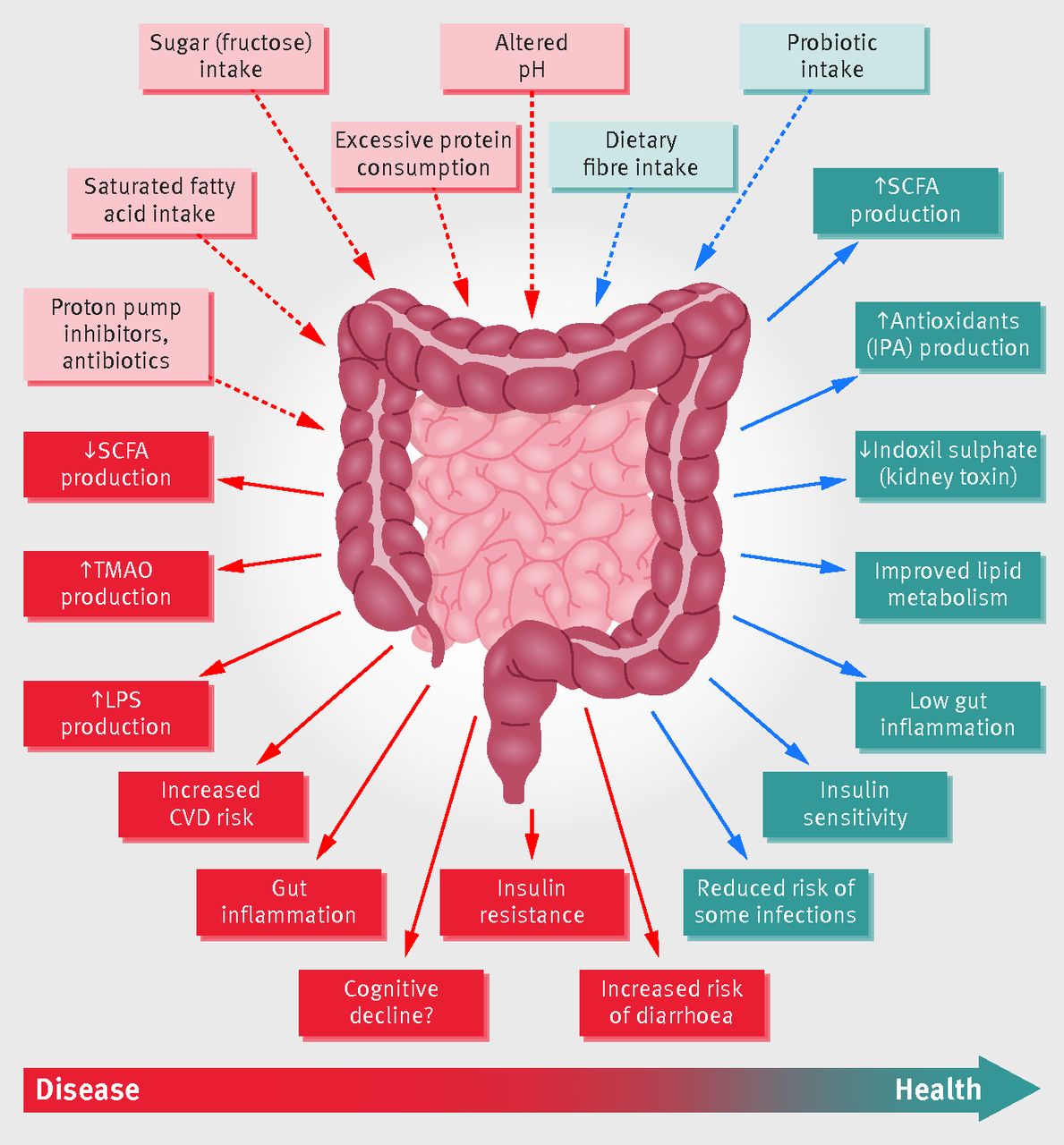 Whilst high-fibre diets are excellent for gut health, there is a suggestion that microbes may experience stress at the point when a sudden change from low- to high-fibre diets takes place. One indicator of such stress is an increase in Enterobacteriaceae, known as a pathogenic species of bacteria. Short-term dietary interventions which increase fibre consumption were shown to cause a slight but significant decrease in diversity, possibly associated with a slight but, once again, significant increase in Enterobacteriaceae – a species which is typically lower in vegan compared with omnivorous diets 58 .
Whilst high-fibre diets are excellent for gut health, there is a suggestion that microbes may experience stress at the point when a sudden change from low- to high-fibre diets takes place. One indicator of such stress is an increase in Enterobacteriaceae, known as a pathogenic species of bacteria. Short-term dietary interventions which increase fibre consumption were shown to cause a slight but significant decrease in diversity, possibly associated with a slight but, once again, significant increase in Enterobacteriaceae – a species which is typically lower in vegan compared with omnivorous diets 58 .
This is probably the case because of the fact that longer-term dietary habits that favour high-fibre foods are known to produce greater amounts of butyrate-producing bacteria, which lower the colonic pH and, thus, prevent the growth of pathogenic bacteria, such as Enterobacteriaceae 59 .
Obesity & reduced microbial diversity
Studies have shown 60 reduced microbial diversity in obese individuals. In addition, it was shown that obese individuals have a reduction in the Bacteriodetes:Firmicutes ratio – that is, increased numbers of Bacteriodetes and decreased numbers of Firmicutes (more on this ratio below), an increase in Proteobacteria (a pro-inflammatory phylum), and an increase in C-reactive protein (which is inversely correlated with a healthy Bacteriodetes:Firmicutes ratio – that is, more C-reactive protein means a less favourable Bacteriodetes:Firmicutes ratio).
It’s possible to see quite the opposite results when looking at the ~60,000 participants in the Adventist Health Study-2 61 . The individuals who followed a vegan diet shown the lowest BMI values when compared with vegetarians or omnivores. The suggestion followed that the lower body weight associated with vegan diets might produce a microbial diversity which protects against systemic inflammation.
Why the Bacteroidetes:Firmicutes ratio matters
“Out of thousands of bacterial species-level phylotypes 62 inhabiting the human gut, the majority belong to two dominant phyla, the Bacteroidetes and Firmicutes . Members of the Bacteroidetes in particular have been associated with human metabolic diseases.” 63 So, even taking into account the wide range of inter-individual variations in human intestinal microbiomes, it’s known 64 65 that these Bacteroidetes and Firmicutes phyla dominate healthy human microbiomes.
However, it’s the actual ratio between them that seems to matter most. Diets heavier in animal fat and protein than in whole grains and plant-based foods (rich in starch, fibre and plant protein) have been shown 66 to cause a significant decrease in Bacteroidetes and increase in Firmicutes.
See the following video by Dr Greger for more information on this important ratio:
In the video, Dr Greger offers a useful way of remembering which bacterial phyla is associated with which body type: Bacteroidetes for bony, and Firmicutes for fat!
Bacteroidetes:Firmicutes ratio and obesity
As the above video explained, a decrease in Firmicutes levels usually favours an increase in Bacteroidetes and Bifidobacteria – something that happens when you eat lots of resistant starches 67 . The typical result of this is that obesity/high BMI can be prevented and treated 68 .
A decreased Bacteroidetes:Firmicutes ratio has a strong negative correlation with BMI 60 . This may be explained by the observation that a 20% increase in Firmicutes with a corresponding decrease in Bacteroidetes abundance is associated with a 150 kcal/day increase in energy harvest 69 – something which would, over time, result in weight gain. Thus, an increased Bacteroidetes:Firmicutes ratio (as seen in high-fibre, plant-based diets) could result in weight loss by causing a reduction in the amount of energy being extracted from the diet.
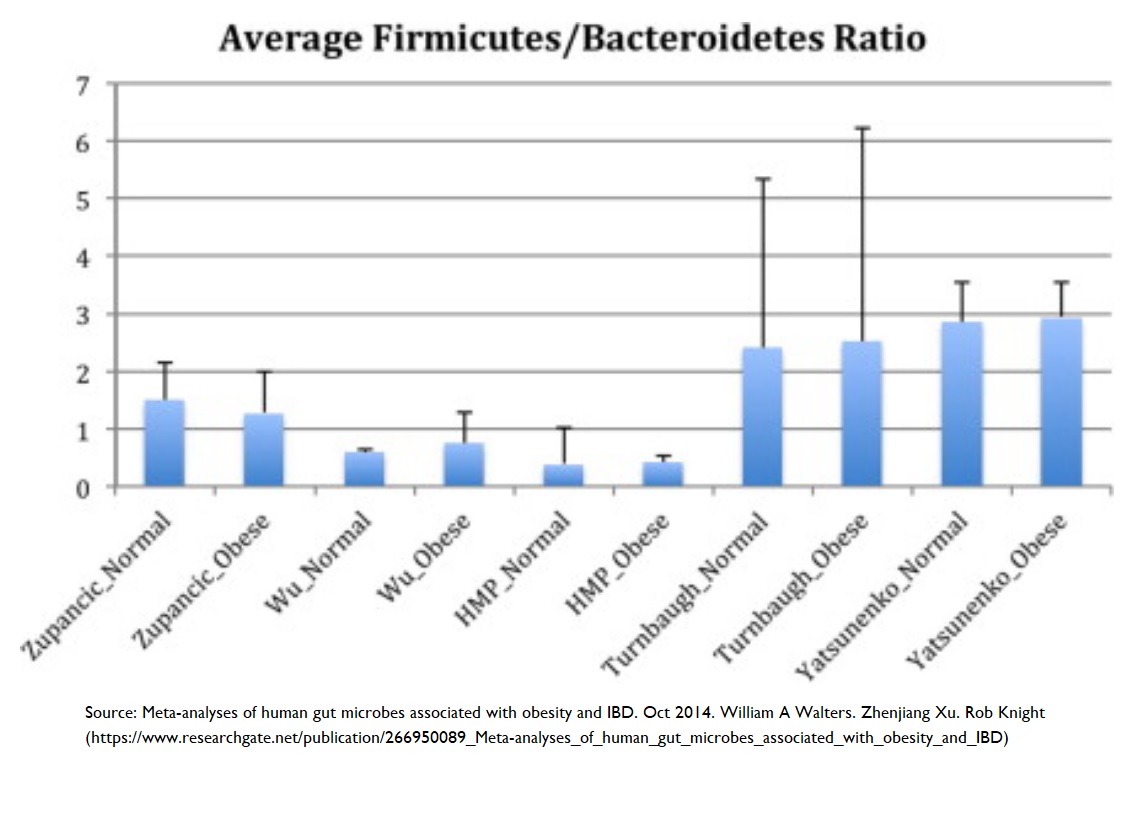
Additional studies are required to decide whether increased energy harvest is caused by the Bacteroidetes:Firmicutes ratio promoting adiposity or by a host-mediated adaptive response to limit energy uptake 70 .
Nothing is plain and simple in the world of microbes, and there are sufficient conflicting results from various studies on the Bacteroidetes:Firmicutes ratio to support the argument that making wide-sweeping statements about which phyla are good and which are bad is likely to be inaccurate and over-generalised. Increased awareness is growing about a need to appreciate the complexity of dynamic interactions between microbes within the microbiome, rather than trying to match one microbe with one health outcome 71 52 72 73 .
How gut bacteria enterotypes are affected by diet
As mentioned above, there are three main enterotypes observed in human microbiomes:
1. Prevotella
2. Bacteroides
3. Ruminococcus
1. Prevotella is a genus of the Bacteroidetes phyla. Studies suggest 74 75 76 77 that it’s significantly richer in the microbiome of vegans.
Studies with mice have suggested 78 that Prevotella improves glucose metabolism by improving glycogen storage. It has also been observed 79 to confer anti-inflammatory effects, and additional research suggests 80 that it can decrease the growth of other bacteria by competing for fibre as an energy substrate.
2. Bacteroides is another main enterotype and genus of the Bacteroidetes phyla. Whilst still appearing to be affected by the type of diet eaten, Bacteroidetes has been positively associated 81 52 with long-term diets high in saturated fat and animal protein.
An explanation of this is that such bacteria are better able to tolerate bile – something common in the gut environments of meat-eaters. It’s no surprise, therefore, that high proportions of Bacteroides are found 76 in the intestinal environments of those who eat the modern Western diet, while the opposite is the case for those eating lots of legumes, fruits and fibre.
3. Ruminococcus is an enterotype and genus also associated with long-term fruit and vegetable consumption. An explanation of this is that species of this genus are specialists in degrading complex carbohydrates (e.g. cellulose and resistant starch which are only found in plants) and, thereby, producing butyrate, an anti-inflammatory SCFA56 .

Increased communities of Ruminococcus have been associated with the following:
“A high fat diet increases the absorption of LPS [lipopolysaccharides or LPS are an integral component of Gram negative microorganisms], which, in turn, has been found to be associated with metabolic endotoxemia and to induce inflammation resulting in obesity.” 83
Walnuts, refined grains and Ruminococcus
Studies have shown 65 that eating walnuts appears to enrich the Ruminococcus community within our gut environment.
Interestingly, however, one study showed 84 an increase of Ruminococcus in college students who had low dietary fibre intake in their diets. This may be explained by the ability of these bacteria to also break down resistant starches in the many refined grain products eaten by this group.
How plant food components influence gut microbiota
This final section will look at both nutrient bioavailability and the microbial responses to the consumption of carbohydrates.
Nutrient Bioavailability
It may seem counter-intuitive, but less is more when it comes to certain aspects of nutrient bioavailability85 . When you consume food nutrients with low bioavailability (normally found in intact plant cell walls, larger food particles, and the latter foods without thermal treatment), more nutrients are able to pass through the stomach and small intestine to reach lower in the gastrointestinal tract. This is important since it allows our gut microbiota, lurking down in the colon, to receive a delivery of rich nutrients 86 .
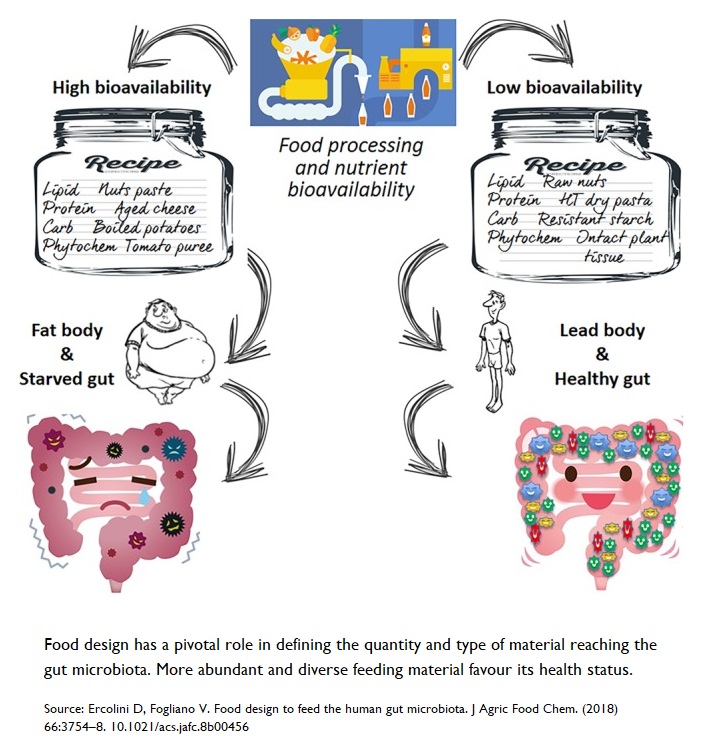
A rich supply of nutrients will help support the normal development and functions of gut microbiota. The modern Western diet has loads of ultra-processed foods and acellular nutrients87 . The small intestine can more easily absorb these components and, thus, deprive the colon of important nutrients. Eating acellular food has been shown 88 to alter the composition and metabolism of the gut microbiota, and induce inflammation in young infants, adolescents, women of child-bearing age, and older adults.
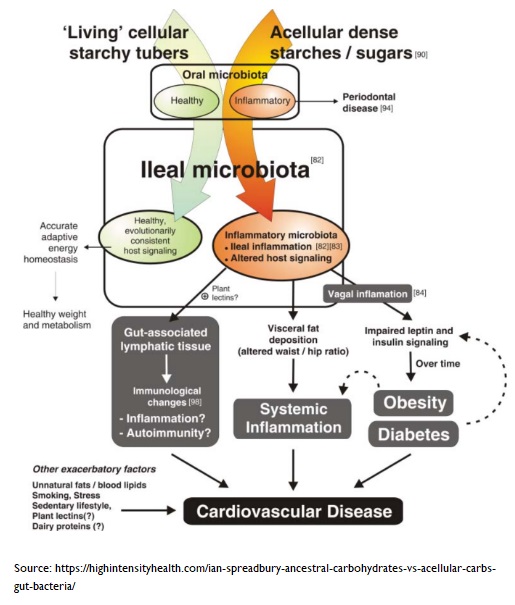
On the other hand, whole plant foods have protective effects, and favour the growth of beneficial fibre-degrading bacteria in the colon.
Carbohydrates
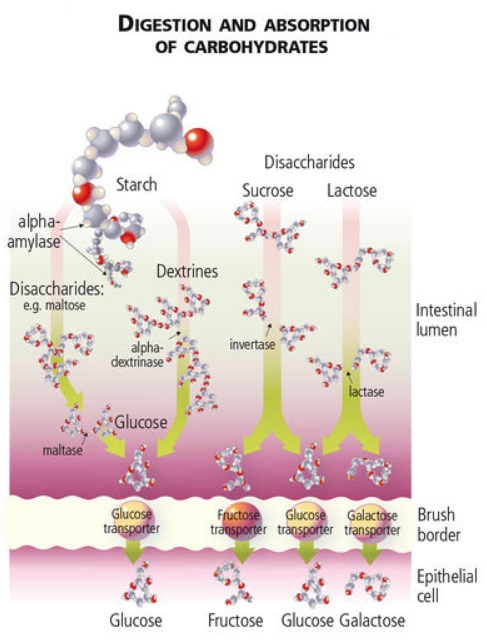
Both digestible and non-digestible carbohydrates appear able to influence gut microbiota.
Digestible carbohydrates (e.g. glucose, sucrose, and fructose from fruits) have been shown 89 to reduce the following:
The following diagram 92 provides more detail on the absorption of digestible carbohydrates:
Non-digestible carbohydrates (NDCs) generally:
- increase the following:
- lactic acid bacteria
- Ruminococcus
- Eubacterium rectale
- Roseburia,
- reduce the following:
- Clostridium
- Enterococcus
The following diagram 93 provides a little more in-depth detail on NDCs.
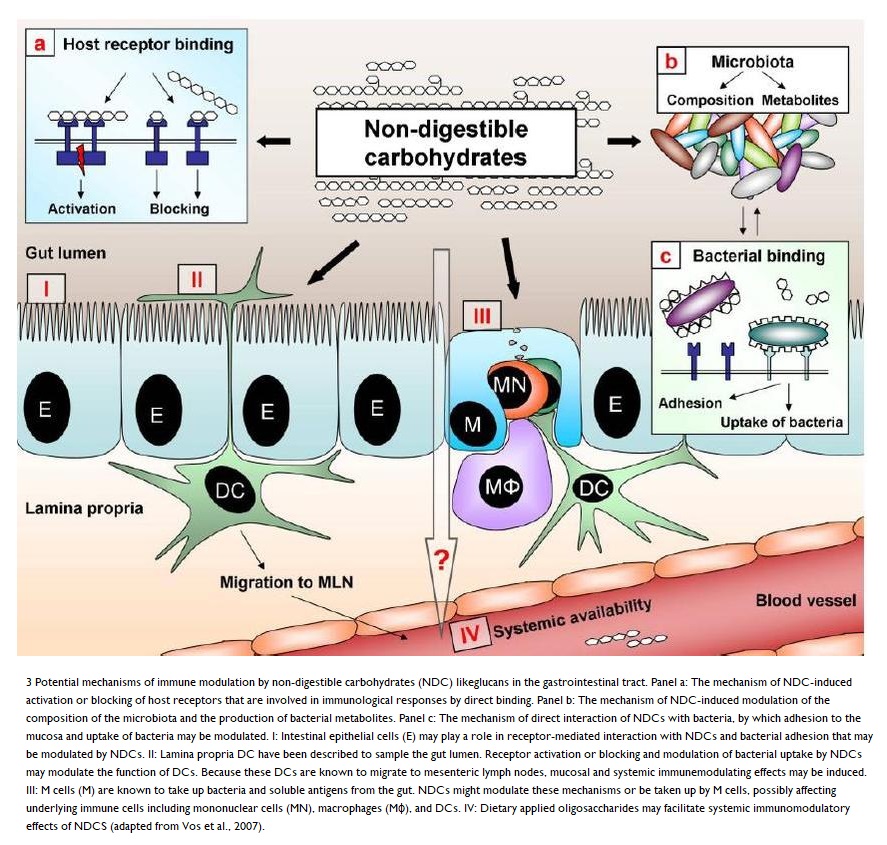
Both digestible and non-digestible carbohydrates have been shown to increase Bifidobacteria (a genus of the Actinobacteria phylum)
Bifidobacteria are some of the good guys. As a butyrate-producing genus, with known protective properties for the human gut barrier, Bifidobacteria provide effective defences against pathogens and diseases, and increase in number when “fed” with health-giving short-chain fructooligosaccharides (scFOS) and fibre – both forms of carbohydrate found in abundance in natural plant foods, such as bananas, artichokes, onions, etc 94 . On the other hand, high consumption of cholesterol (only found in animal foods), was strongly associated with a lower abundance of Bifidobacteria 95 .
Prebiotic fibres
A recent study found 96 that the following fibres have differing prebiotic effects on gut microbiota:
- inulin
- alpha-linked galacto-oligosaccharides
- beta-linked galacto-oligosaccharides
- xylo-oligosaccharides (from corn cobs and high-fibre sugar cane)
- beta-glucan (from oats)
Inulin and all oligosaccharides have a strong bifidogenic effect 97 .
Beta-glucan induces Prevotella and Roseburia growth with associated increase in production of the SCFA, propionate – a good thing, since it’s known 98 99 to promote weight loss and provide many other health benefits, including being anti-carcinogenic and anti-inflammatory.
All natural sugars, especially non-digestible forms like inulin and oligosaccharides, increase SCFA levels 100 101 .
Prebiotic effects vary depending on which type of bacteria breaks down which type of fibre. The specificity of bacterial activity in relation to fibre is determined by specific gene clusters within the bacterial genome that dictate which saccharolytic 102 enzymes the bacteria can produce and, hence, whether they can metabolise the particular prebiotic substrate 103 .
Other protective effects of non-digestible carbohydrates
NDCs don’t just act as prebiotics by promoting the growth of beneficial microorganisms, they are also shown 89 to do the following:
- reduce proinflammatory cytokine 104 production
- reduce concentrations of serum triglycerides
- reduce total cholesterol
- reduce LDL-cholesterol
It can be seen from the above that NDCs are, thus, likely to confer significant protective effects against cardiovascular disease as well as central nervous system disorders.
Final thoughts
An increasing body of research indicates that diet is the essential factor when it comes to the composition of human gut microbiota. These microbes are then responsible for converting nutrients into active postbiotics that we (the hosts) absolutely need. The weight of evidence is favoured towards a plant-based diet being the most effective way in which we can ensure a diverse ecosystem of beneficial microbes is promoted within us, so that they can support overall health.
References & Notes
- Alcohol – Bad News for Good Bacteria [↩]
- Obstructive Sleep Apnea (OSA) & Gut Microbiota [↩]
- Gut Microbiota & Depression [↩]
- Physical Activity for Disease Prevention & Healthy Gut Microbiome [↩]
- Multiple Sclerosis (MS), Serotonin & Gut Microbiota [↩]
- The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, Kahleova H. Front Nutr. 2019 Apr 17;6:47. doi: 10.3389/fnut.2019.00047. eCollection 2019. Review. PMID: 31058160. [↩]
- see below for definition of “postbiotics”. [↩]
- Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. (2016) 14:e1002533. 10.1371/journal.pbio.1002533 [↩]
- Functional redundancy is a characteristic of species within an ecosystem where certain species contribute in equivalent ways to an ecosystem function such that one species may substitute for another. [↩]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. . A human gut microbial gene catalogue established by metagenomic sequencing. Nature. (2010) 464:59–65. 10.1038/nature08821. [↩]
- Two Types of Gut Bacteria: Plant Eaters’ & Meat Eaters’ [↩]
- Cheeseburger vs Tofu Burger for Gut Health & Satiety [↩]
- Butyrate – Why Dietary Fibre is So Important [↩]
- Broccoli & Sulforaphane vs Cancer [↩]
- How to Reduce Your TMAO Levels [↩]
- How to Reduce Carcinogenic Bile Acid Production [↩]
- Derrien M, Veiga P. Rethinking diet to aid human-microbe symbiosis. Trends Microbiol. (2017) 25:100–12. 10.1016/j.tim.2016.09.011 [↩]
- Wong MW, Yi CH, Liu TT, Lei WY, Hung JS, Lin CL, et al. . Impact of vegan diets on gut microbiota: an update on the clinical implications. Tzu Chi Med J. (2018) 30:200–3. 10.4103/tcmj.tcmj_21_18 [↩]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. . Enterotypes of the human gut microbiome. Nature. (2011) 473:174–80. 10.1038/nature09944 [↩]
- Roager HM, Licht TR, Poulsen SK, Larsen TM, Bahl MI. Microbial enterotypes, inferred by the prevotella-to-bacteroides ratio, remained stable during a 6-month randomized controlled diet intervention with the new nordic diet. Appl Environ Microbiol. (2014) 80:1142–9. 10.1128/AEM.03549-13. [↩]
- An enterotype is a classification of living organisms based on its bacteriological ecosystem in the gut microbiome. The discovery of three human enterotypes was announced in the April 2011 issue of Nature by Peer Bork and his associates. [↩]
- Bull MJ, Plummer NT. Part 1: The human gut microbiome in health and disease. Integr Med Clin J. (2014) 13:17–22. [↩]
- Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. (2009) 136:65–80. 10.1053/j.gastro.2008.10.080 [↩]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. (2009) 9:313–23. 10.1038/nri2515 [↩]
- Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. (2011) 9:279–90. 10.1038/nrmicro2540 [↩]
- Cancer J. Author manuscript; available in PMC 2015 May 1. Microbiome in Reflux Disorders and Esophageal Adenocarcinoma. Liying Yang, MD, Noami Chaudhary, MD, Jonathan Baghdadi, MD, and Zhiheng Pei, MD, PhD. [↩]
- Gut Microbes. 2013 Nov 1; 4(6): 505–531. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Alexander Sheh, James G Fox. [↩]
- Version 1. F1000Res. 2018; 7: F1000 Faculty Rev-1029. The gut microbiome and irritable bowel syndrome. Stacy Menees et al. [↩]
- Cell Mol Life Sci. 2019 Apr;76(8):1541-1558. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Safari Z, Gérard P. [↩]
- Microbiol., 25 September 2018. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Tao Zuo, Siew C. Ng. [↩]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. (2006) 444:1022–3. 10.1038/4441022a [↩]
- Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. (2017) 14:79–87. 10.1038/nrcardio.2016.183 [↩]
- Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. . Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. (2010) 5:e9085. 10.1371/journal.pone.0009085 [↩]
- Jiang C, Li G, Huang P, Liu Z, Zhao B. The gut microbiota and Alzheimer’s Disease. J Alzheimers Dis JAD. (2017) 58:1–15. 10.3233/JAD-161141 [↩]
- Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, et al. . Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. (2016) 32:66–72. 10.1016/j.parkreldis.2016.08.019 [↩]
- Physiol Rep. 2017 Sep; 5(18): e13443. Gut inflammation and dysbiosis in human motor neuron disease. Julie Rowin, Yinglin Xia, Barbara Jung, Jun Sun. [↩]
- Biol Res Nurs. 2018 Oct;20(5):513-521. doi: 10.1177/1099800418784202. Epub 2018 Jun 20. Potential Role of the Gut Microbiome in ALS: A Systematic Review. Wright ML et al. [↩]
- Ding HT, Taur Y, Walkup JT. Gut microbiota and autism: key concepts and findings. J Autism Dev Disord. (2017) 47:480–9. 10.1007/s10803-016-2960-9 [↩]
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, et al. . Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. (2015) 138:179–87. [↩]
- Atopy refers to the genetic tendency to develop allergic diseases such as allergic rhinitis, asthma and atopic dermatitis (eczema). Atopy is typically associated with heightened immune responses to common allergens, especially inhaled allergens and food allergens. [↩]
- Front Immunol. 2018; 9: 1584. Microbiome and Allergic Diseases. Mariona Pascal et al. [↩]
- Selber-Hnatiw S, Rukundo B, Ahmadi M, Akoubi H, Al-Bizri H, Aliu AF, et al. . Human gut microbiota: toward an ecology of disease. Front Microbiol. (2017) 8:1265. 10.3389/fmicb.2017.01265. [↩]
- What is a good source of probiotics? Written By Michael Greger M.D. FACLM on November 8th, 2012 [↩]
- Culture Shock – Questioning the Efficacy and Safety of Probiotics. Michael Greger M.D. FACLM October 25th, 2017 Volume 38 [↩]
- do Rosario VA, Fernandes R, Trindade EB. Vegetarian diets and gut microbiota: important shifts in markers of metabolism and cardiovascular disease. Nutr Rev. (2016) 74:444–54. 10.1093/nutrit/nuw012 [↩] [↩]
- Losasso C, Eckert EM, Mastrorilli E, Villiger J, Mancin M, Patuzzi I, et al. . Assessing the Influence of Vegan, Vegetarian and Omnivore Oriented Westernized Dietary Styles on Human gut microbiota: a cross sectional study. Front Microbiol. (2018) 9:317. 10.3389/fmicb.2018.00317 [↩]
- Substrates are the reactants, products, and intermediates of an enzymatic reaction, generally known as metabolites, which are modified by a sequence of chemical reactions catalysed by enzymes. In most cases of a metabolic pathway, the product of one enzyme acts as the substrate for the next. [↩]
- Journal of Neurogastroenterology and Motility 2017; 23(1): 124-134. Colonic Transit Time Is a Driven Force of the Gut Microbiota Composition and Metabolism: In Vitro Evidence. William Tottey et al. [↩]
- Alkaline Diet – So What?! [↩]
- Dietary Patterns Affect the Gut Microbiome—The Link to Risk of Cardiometabolic Diseases. Alyssa M Tindall Kristina S Petersen Penny M Kris-Etherton. The Journal of Nutrition, Volume 148, Issue 9, September 2018, Pages 1402–1407. [↩]
- Salonen A, de Vos WM. Impact of diet on human intestinal microbiota and health. Annu Rev Food Sci Technol. (2014) 5:239–62. 10.1146/annurev-food-030212-182554 [↩] [↩]
- Klimenko NS, Tyakht AV, Popenko AS, Vasiliev AS, Altukhov IA, Ischenko DS, et al. . Microbiome responses to an uncontrolled short-term diet intervention in the frame of the citizen science project. Nutrients. (2018) 10:E576. 10.3390/nu10050576 [↩] [↩] [↩] [↩]
- Martínez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, et al. . Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. (2013) 7:269–80. [↩]
- Vascular compliance is the ability of a blood vessel wall to expand and contract passively with changes in pressure is an important function of large arteries and veins. [↩]
- Menni C, Lin C, Cecelja M, Mangino M, Matey-Hernandez ML, Keehn L, et al. . Gut microbial diversity is associated with lower arterial stiffness in women. Eur Heart J. (2018) 39:2390–7. 10.1093/eurheartj/ehy226 [↩] [↩]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. . Diet rapidly and reproducibly alters the human gut microbiome. Nature. (2014) 505:559–63. 10.1038/nature12820 [↩] [↩]
- Zhang C, Björkman A, Cai K, Liu G, Wang C, Li Y, et al. . Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front Immunol. (2018) 9:908. 10.3389/fimmu.2018.00908 [↩]
- Zimmer J, Lange B, Frick JS, Sauer H, Zimmermann K, Schwiertz A, et al. . A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr. (2012) 66:53–60. 10.1038/ejcn.2011.141 [↩]
- Scott KP, Duncan SH, Flint HJ. Dietary fibre and the gut microbiota. Nutr Bull. (2008). 33: 201–211. 10.1111/j.1467-3010.2008.00706.x [↩]
- Verdam FJ, Fuentes S, de Jonge C, Zoetendal EG, Erbil R, Greve JW, et al. . Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obes Silver Spring Md. (2013) 21:E607–615. 10.1002/oby.20466 [↩] [↩]
- Orlich MJ, Fraser GE. Vegetarian diets in the Adventist Health Study 2: a review of initial published findings1234. Am J Clin Nutr. (2014) 100:353S−8S. 10.3945/ajcn.113.071233 [↩]
- A phylotype is a biological type that classifies an organism by its phylogenetic, that is evolutionary, relationship to other organisms. [↩]
- J Mol Med (Berl). 2017; 95(1): 1–8. PMID: 27900395. Microbiome and metabolic disease: revisiting the bacterial phylum Bacteroidetes. Elizabeth L. Johnson, Stacey L. Heaver, William A. Walters, and Ruth E. Ley [↩]
- Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. (2015) 42:158–79. 10.1111/apt.13248 [↩]
- Bamberger C, Rossmeier A, Lechner K, Wu L, Waldmann E, Fischer S, et al. . A walnut-enriched diet affects gut microbiome in healthy caucasian subjects: a randomized, controlled trial. Nutrients. (2018) 10. 10.3390/nu10020244 [↩] [↩]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. . Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. (2010) 107:14691–6. 10.1073/pnas.1005963107 [↩]
- Nutrition Foundation: Resistant Starch. [↩]
- Martínez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PloS ONE. (2010) 5:e15046. 10.1371/journal.pone.0015046 [↩]
- The gut microbiota has emerged as an environmental factor that modulates the host’s energy balance. It increases the host’s ability to harvest energy from the digested food, and produces metabolites and microbial products such as short-chain fatty acids, secondary bile acids, and lipopolysaccharides. [↩]
- Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. (2005) 102:11070–5. 10.1073/pnas.0504978102 [↩]
- Lin A, Bik EM, Costello EK, Dethlefsen L, Haque R, Relman DA, et al. . Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PloS ONE. (2013) 8:e53838. 10.1371/journal.pone.0053838 [↩]
- Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. . Microbiota and SCFA in lean and overweight healthy subjects. Obes Silver Spring Md. (2010) 18:190–5. 10.1038/oby.2009.167 [↩]
- Andoh A, Nishida A, Takahashi K, Inatomi O, Imaeda H, Bamba S, et al. . Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J Clin Biochem Nutr. (2016) 59:65–70. 10.3164/jcbn.15-152 [↩]
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. . Gut microbiota composition correlates with diet and health in the elderly. Nature. (2012) 488:178–84. 10.1038/nature11319 [↩]
- Ruengsomwong S, La-Ongkham O, Jiang J, Wannissorn B, Nakayama J, Nitisinprasert S. Microbial community of healthy thai vegetarians and non-vegetarians, their core gut microbiota, and pathogen risk. J Microbiol Biotechnol. (2016) 26:1723–35. 10.4014/jmb.1603.03057 [↩]
- Jeffery IB, O’Toole PW. Diet-microbiota interactions and their implications for healthy living. Nutrients. (2013) 5:234–252. 10.3390/nu5010234 [↩] [↩]
- Matijašić BB, Obermajer T, Lipoglavšek L, Grabnar I, Avguštin G, Rogelj I. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia. Eur J Nutr. (2014) 53:1051–64. 10.1007/s00394-013-0607-6 [↩]
- Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, et al. . Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab. (2015) 22:971–82. 10.1016/j.cmet.2015.10.001 [↩]
- to confer anti-inflammatory effects. However, perhaps all that can be currently stated is that Prevotella is merely a genus that describes an overall ecosystem of human gut bacteria which is mainly experienced on a plant-based diet. Prevotella has been observed ((De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. . Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. (2010) 107:14691–6. 10.1073/pnas.1005963107 [↩]
- Chen T, Long W, Zhang C, Liu S, Zhao L, Hamaker BR. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci Rep. (2017) 7:2594. 10.1038/s41598-017-02995-4 [↩]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. . Linking long-term dietary patterns with gut microbial enterotypes. Science. (2011) 334:105–8. 10.1126/science.1208344 [↩]
- Endotoxemia is essentially an innate immune response that becomes a sub-clinical, persistent, low- grade inflammation because of increased, circulating endotoxins, primarily LPS or lipopolysaccharides. The presence of such endotoxins in the blood, if derived from gram-negative rod-shaped bacteria, like LPS, may cause haemorrhages, necrosis of the kidneys, and shock. Metabolic endotoxemia is a condition that is estimated to affect approximately 33% of the western population. [↩]
- World J Gastrointest Pathophysiol. 2015 Nov 15; 6(4): 110–119. New-found link between microbiota and obesity. Chandra Kanti Chakraborti. [↩]
- Whisner CM, Maldonado J, Dente B, Krajmalnik-Brown R, Bruening M. Diet, physical activity and screen time but not body mass index are associated with the gut microbiome of a diverse cohort of college students living in university housing: a cross-sectional study. BMC Microbiol. (2018) 18:210 10.1186/s12866-018-1362-x [↩]
- Several definitions exist, but nutrient bioavailability is broadly defined as the proportion of a nutrient that is absorbed from the diet and used for normal bodily functions. [↩]
- Ercolini D, Fogliano V. Food design to feed the human gut microbiota. J Agric Food Chem. (2018) 66:3754–8. 10.1021/acs.jafc.8b00456 [↩]
- Acellular nutrients are those which do not have any cells, such as refined flour, processed grains and white sugar [↩]
- Zinöcker MK, Lindseth IA. The western diet-microbiome-host interaction and its role in metabolic disease. Nutrients. (2018) 10:3. 10.3390/nu10030365 [↩]
- Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, et al. . Influence of diet on the gut microbiome and implications for human health. J Transl Med. (2017) 15:73. 10.1186/s12967-017-1175-y [↩] [↩]
- Bacteroides fragilis has two main traits that allow it to switch from good to bad – an ability to easily incorporate genes shared by other bacteria and an ability to turn specific genes ‘on’ or ‘off’ as needed. Combined, these traits allow these bacteria to exploit new nutrition pathways, protecting themselves from toxic substrates, and changing the molecules expressed on its
surface. This ‘commensal chameleon’ is the perfect opportunistic pathogen. [↩] - Clostridia are an anaerobic class of Firmicutes. Clostridia are members of normal human flora, present primarily in the intestinal tract and vagina, and are ubiquitous in soil. Generally safe in situ but dangerous if spread through other parts of the body [↩]
- Wikivet: Digestibility of Carbohydrates [↩]
- 3 Potential mechanisms of immune modulation by non-digestible carbohydrates. Research Gate. [↩]
- J Physiol Biochem. 2009 Sep;65(3):315-28. doi: 10.1007/BF03180584. Dietary fructooligosaccharides and potential benefits on health. Sabater-Molina M1, Larqué E, Torrella F, Zamora S. [↩]
- Whelan K, Judd PA, Preedy VR, Simmering R, Jann A, Taylor MA. Fructooligosaccharides and fiber partially prevent the alterations in fecal microbiota and short-chain fatty acid concentrations caused by standard enteral formula in healthy humans. J Nutr. (2005) 135:1896–902. 10.1093/jn/135.8.1896 [↩]
- Fehlbaum S, Prudence K, Kieboom J, Heerikhuisen M, van den Broek T, Schuren FHJ, et al. . In vitro fermentation of selected prebiotics and their effects on the composition and activity of the adult gut microbiota. Int J Mol Sci. (2018) 19:10. 10.3390/ijms19103097 [↩]
- The bifidogenic effect is where a bifidus factor (bifidogenic factor) specifically enhances the growth of bifidobacteria in either a product or in the intestines of humans and/or animals. Several products have been marketed as bifidogenic factors, such as several prebiotics and methyl-N-acetyl D-glucosamine in human milk. [↩]
- Nutr Rev. 2011 May;69(5):245-58. Propionate as a health-promoting microbial metabolite in the human gut. Hosseini E, Grootaert C, Verstraete W, Van de Wiele T. [↩]
- Pharmacol Ther. 2016 Aug;164:144-51. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Sivaprakasam S, Prasad PD, Singh N. [↩]
- Nutrients. 2013 Apr; 5(4): 1417–1435. Fiber and Prebiotics: Mechanisms and Health Benefits. Joanne Slavin. [↩]
- J Zhejiang Univ Sci B. 2009 Apr; 10(4): 258–263. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. Xiao-dong Pan, Fen-qin Chen, Tian-xing Wu, Hong-gang Tang, and Zhan-yu Zhao. [↩]
- Saccharolytic enzymes are capable of hydrolysing or otherwise breaking down a sugar molecule. [↩]
- Wilson B, Whelan K. Prebiotic inulin-type fructans and galacto-oligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J Gastroenterol Hepatol. (2017) 32:64–8. 10.1111/jgh.13700 [↩]
- Cytokines are any of a number of substances, such as interferon, interleukin, and growth factors, which are secreted by certain cells of the immune system and have an effect on other cells. [↩]