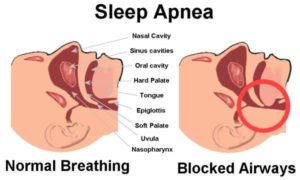
It might not seem intuitively obvious, but if you have trouble breathing when you sleep (known as OSA or obstructive sleep apnea), it may not be just the you who think you are who is suffering. Increasing evidence suggests that the trillions of bacteria that live inside you (or, perhaps, just as accurately, the trillions of bacteria that you live around) are also inextricably involved in your night-time gasps for breath. And the net result could be a whole range of diseases, from hypertension and obesity to cardiovascular disease.
Blog Contents
New field of study
This area of research is relatively new. Indeed, the whole field of gut (as well as urinary and oral) bacteria (known as the microbiota or collectively as the microbiome) is something of an emerging journey into a vast and previously unknown world. I’ve covered quite a lot of issues regarding microbiota and other related factors already – depression 1 2 , multiple sclerosis 3 , physical activity 4 , fibromyalgia 5 , meat-eaters vs plant-eaters 6 7 , and overactive bladder 8 . But this short blog just aims to bring to your mind the possibility that the bacteria that call you their home don’t sleep when you do – or when you don’t!
A brief look at the research
The following is a brief chronological review of some of the research in this area of sleep and microbiota.
A 2013 study 9 concludes: “It is expected that the improvement of sleep quality after exercise and diet intervention will be evident both in subjective and objective measures of quality of sleep. Additionally, the change of sleep quality induced by exercise and diet intervention is expected to be related to the changes in specific hormones and inflammatory biomarkers, and in the composition of gut microbiota.”
This is interesting because we already know that dietary changes are linked to changes in the gut microbiota. It’s therefore not such a leap to expect that the general quality of sleep (and that’s what this study deals with, rather than specifically OSA) is affected by the type and quantity of little guys working away in our guts. And the knock on effects for our health, in terms of the hormonal and inflammatory changes that our microbiota can cause, can be significant.
A February 2016 study 10 considered how individuals who suffer from OSA are therefore at increased risk for systemic hypertension.
They view the importance on the host physiology (you and me) of a healthy gut microbiota, and the detrimental effects of a dysbiotic microbiota, are becoming increasingly evident. They started from the hypothesis that gut dysbiosis contributes to the incidences of both hypertension and OSA existing in the same individuals.
They used rats and inflated a tracheal balloon during their sleep cycle (not a nice thought if you’re a rat!). On a normal diet, the resulting OSA had no effect on blood pressure; however, in when the rats were fed a high-fat diet, their blood pressure increased significantly (24 and 29 mm Hg after 7 and 14 days of OSA). They then looked at the bacteria in the rats’ faecal pellets and found that high-fat diet and OSA led to significant alterations of the gut microbiota, including decreases in bacterial taxa known to produce the short chain fatty acid butyrate 11 . A really interesting step they then took was to transplant dysbiotic faecal contents from hypertensive OSA rats on high-fat diet into OSA recipient rats on normal diets. And the results? The recipient rats started to show similar levels of hypertension to that of the donors (increased 14 and 32 mm Hg after 7 and 14 days of OSA).
This fascinating and revealing study concludes that: “These studies demonstrate a causal relationship between gut dysbiosis and hypertension, and suggest that manipulation of the microbiota may be a viable treatment for OSA-induced, and possibly other forms of, hypertension.”
This is the sort of science I love, although I can’t speak for the poor rats!
A February 2017 study 12 , which is a little ‘off piste’ is still interesting. It was looking at the recently reported association between paediatric obstructive sleep apnea syndrome (OSAS) and Group A streptococcus (GAS) sub-acute chronic tonsil colonisation.
This is relevant, as you would possibly know from previous blogs, because the bacteria that live with us are not confined to our intestines. They are everywhere – including the mouth, throat and, in relation to this study, specifically the tonsils. And their fundamentally important relationship with our health is becoming increasingly apparent – as can be seen from this particular study.
They found an association between the slaA gene (in Streptococcus) and the GAS OSAS strains only in patients affected by OSAS, thus suggesting that this genotype might be associated to the pathogenesis of OSAS.
A November 2017 study 13 looked at the possibility that the comorbidity of OSA and atheroscleriosis are linked to gut microbiota. The researchers started from the premise that there is an established link between OSA and cardiovascular morbidity and mortality (including myocardial infarction, stroke, and peripheral artery disease). The latter are seen as consequences of atherosclerosis, itself an inflammatory condition brought about by chronic intermittent hypoxia (IH) and sleep fragmentation (SF). The researchers come to the conclusion that all the foregoing conditions are linked to our gut bacteria – a sort of feedback loop that, if untreated, may lead to unhealthy gut bacteria and unpleasant, even fatal, diseases operating together to the detriment of the host.
Their findings are consistent with other studies 14 15 that reported potential benefits of continuous positive airway pressure treatment for cardiovascular disease in patients with OSA.
Following on from the above study 12 which considered OSA in relation to streptococcus around the tonsils, a December 2017 study 16 also looked down the throats of OSA sufferers to see if there was any difference between the type of adenoidal microbiota from two groups of patients, one with recurrent acute otitis media (AOM) 17 and one with OSA. They concluded that the type of microbiota was different between the two groups. My assumption is that there would be differences between the adenoidal microbiota of both of the latter groups and those individuals who have neither condition.
An updated review in June 2018 18 considers adenotonsillar microbiota and describes the new techniques applied in determining the nature of the microbiome and summarises the results of studies to date in this field.
A March 2018 study 19 takes up the above-mentioned issue of feedback (termed “cross-talk” by the authors) between our bacteria and our bodies, where the health of one can affect the health of the other.
This study looked at how OSA has emerged as: “…a highly prevalent public health problem that imposes important mid- and long-term consequences, namely cardiovascular, metabolic, cognitive, and cancer-related alterations.”
They point out that OSA is characterised by increased upper airway resistance, alveolar hypoventilation 20 and recurrent upper airway obstruction during sleep. “Recurrent collapse of the upper airway develops with sleep onset and is associated with both intermittent hypoxemia and sleep fragmentation.”
This is one study that does point out that lifestyle factors can affect changes in gut microbial communities. Such lifestyle factors as:
- smoking
- long-distance travel
- dietary preferences
- physical exercise, and
- circadian rhythm disturbances
Parallel to this, they point out that diseases previously attributed in part to lifestyle such as obesity, coronary heart disease, depression, and asthma are associated with OSA.
Furthermore (to complete the circle) they emphasise that OSA is related to microbiota.
They conclude that: “…altered patterns of sleep and oxygenation, as seen in OSA, will promote specific alterations in gut microbiota that in turn will elicit the immunologic alterations that lead to OSA-induced end-organ morbidities 21 .”
The last research we’ll look at is a June 2018 study 22 which explained how OSA is a “common disorder characterized by episodic obstruction to breathing due to upper airway collapse during sleep. Because of the episodic airway obstruction, intermittently low O2 (hypoxia) and high CO2 (hypercapnia) ensue. OSA has been associated with adverse cardiovascular and metabolic outcomes, although data regarding potential causal pathways are still evolving. As changes in inspired O2 and CO2 can affect the ecology of the gut microbiota and the microbiota has been shown to contribute to various cardiometabolic disorders, we hypothesized that OSA alters the gut ecosystem, which, in turn, exacerbates the downstream physiological consequences.”
The experiments they did were on mice fed a high-fat diet and exposed to intermittent hypoxia and hypercapnia (known together as IHH).
The results of their experiments showed that there were “marked compositional changes in both microbial (>10%, most remarkably in Clostridia 23 ) and molecular (>22%) species in the gut. Moreover, molecules that altered in abundance included microbe-dependent bile acids, enterolignans 24 , and fatty acids, highlighting the impact of IHH on host-commensal organism cometabolism in the gut”.
This was the first evidence that IHH messes with gut microbiota in a functional manner. The implication of this is that it starts to provide explanations for the comorbidity (diseases existing together) of OSA and various cardiovascular diseases. When you have a microbiota that’s impaired or out of balance (referred to as intestinal dysbiosis), the scene is set for the development of diseases, including cardiovascular and metabolic diseases, actually caused by OSA.
The researchers point out that much more research is needed, but they seem pretty confident that there are as yet unrecognised mechanistic links between gut microbes and comorbidities of OSA.
Joe’s Final Comment
The foregoing seems to indicate that our bacteria appear inextricably connected to a wide range of medical conditions. How effective it might be in avoiding or treating such conditions by making changes to our diet is outside of the auspices of most of the studies covered above – although I suspect it will not be long before a wealth of research studies assess dietary influences on these and many more diseases. Whether they conclude that a plant-based diet is an essential component of any optimally healthy lifestyle is yet to be seen; but, for the time being, I’m taking no chances. For me, there’s already ample evidence that the best way to help our good bacteria to help us to stay well, is to supply them with a varied diet of whole plant foods.
References
- Gut Microbiota & Depression [↩]
- Depression is Linked to Inflammation [↩]
- Multiple Sclerosis (MS), Serotonin & Gut Microbiota [↩]
- Physical Activity for Disease Prevention & Healthy Gut Microbiome [↩]
- Fibromyalgia, Probiotics & Gut Microbiota [↩]
- Two Types of Gut Bacteria: Plant Eaters’ & Meat Eaters’ [↩]
- Oral Microbiota – Meat-Eaters & Plant-Eaters [↩]
- Urinary Microbiome & Psychological Issues in Women with Overactive Bladder [↩]
- Trials. 2013 Jul 26;14:235. doi: 10.1186/1745-6215-14-235. Effects of exercise and diet interventions on obesity-related sleep disorders in men: study protocol for a randomized controlled trial. Tan X, Saarinen A, Mikkola TM, Tenhunen J, Martinmäki S, Rahikainen A, Cheng S, Eklund N, Pekkala S, Wiklund P, Munukka E, Wen X, Cong F, Wang X, Zhang Y, Tarkka I, Sun Y, Partinen M, Alen M, Cheng S. [↩]
- Role of the Gut Microbiome in Obstructive Sleep Apnea-Induced Hypertension. Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, Hollister EB, Bryan RM Jr. Hypertension. 2016 Feb;67(2):469-74. doi: 10.1161/HYPERTENSIONAHA.115.06672. Epub 2015 Dec 28. PMID: 26711739 [↩]
- Butyrate – Why Dietary Fibre is So Important [↩]
- Prevalence of M75 Streptococcus pyogenes Strains Harboring slaA Gene in Patients Affected by Pediatric Obstructive Sleep Apnea Syndrome in Central Italy. Viciani E, Montagnani F, Tordini G, Romano A, Salerni L, De Luca A, Ruggiero P, Manetti AG. Front Microbiol. 2017 Feb 28;8:294. doi: 10.3389/fmicb.2017.00294. eCollection 2017. PMID: 28293224 [↩] [↩]
- Obstructive Sleep Apnea and Atherosclerosis: Both the Gut Microbiome and Hypercapnia Matter. Almendros I, Farré N. Am J Respir Cell Mol Biol. 2017 Nov;57(5):501-503. doi: 10.1165/rcmb.2017-0253ED. PMID: 29090956 [↩]
- Sage Journals: Efficacy of Continuous Positive Airway Pressure Treatment in Patients with Cardiac Arrhythmia and Obstructive Sleep Apnea: What is the Evidence? Asmaa M Abumuamar, Tatyana Mollayeva, Paul Sandor, David Newman, Kumaraswamy Nanthakumar, Colin M Shapiro. First Published November 3, 2017. [↩]
- J Thorac Dis. 2016 Dec; 8(12): E1644–E1646. doi: 10.21037/jtd.2016.12.36. PMCID: PMC5227286. PMID: 28149603. Continuous positive airway pressure therapy and cardiovascular outcomes in obstructive sleep apnoea syndrome: where are we now? Walter T. McNicholas. [↩]
- The Adenoid Microbiome in Recurrent Acute Otitis Media and Obstructive Sleep Apnea. Dirain CO, Silva RC, Collins WO, Antonelli PJ. J Int Adv Otol. 2017 Dec;13(3):333-339. doi: 10.5152/iao.2017.4203. PMID: 29360088. [↩]
- Acute otitis media (A)M): Inflammation of the middle ear in which there is fluid in the middle ear accompanied by signs or symptoms of ear infection: a bulging eardrum usually accompanied by pain; or a perforated eardrum, often with drainage of purulent material (pus [↩]
- Adenotonsillar microbiome: an update. Johnston JJ, Douglas R. Postgrad Med J. 2018 Jun 8. pii: postgradmedj-2018-135602. doi: 10.1136/postgradmedj-2018-135602. [Epub ahead of print] Review. PMID: 29884749 [↩]
- Sleep Apnea Morbidity: A Consequence of Microbial-Immune Cross-Talk? Farré N, Farré R, Gozal D. Chest. 2018 Mar 14. pii: S0012-3692(18)30405-7. doi: 10.1016/j.chest.2018.03.001. [Epub ahead of print] Review. PMID: 29548630. [↩]
- Alveolar hypoventilation is defined as insufficient ventilation leading to hypercapnia, which is an increase in the partial pressure of carbon dioxide as measured by arterial blood gas analysis (PaCO2 [↩]
- End-organ: The ultimately affected organ in a chain of events, such as a disease process (pathophysiology) or a drug’s mechanism of action – sometimes called a target organ in this sense [↩]
- Intermittent Hypoxia and Hypercapnia, a Hallmark of Obstructive Sleep Apnea, Alters the Gut Microbiome and Metabolome. Tripathi A, Melnik AV, Xue J, Poulsen O, Meehan MJ, Humphrey G, Jiang L, Ackermann G, McDonald D, Zhou D, Knight R, Dorrestein PC, Haddad GG. mSystems. 2018 Jun 5;3(3). pii: e00020-18. doi: 10.1128/mSystems.00020-18. eCollection 2018 May-Jun. PMID: 29896566 [↩]
- Clostridium is a genus of Gram-positive bacteria, which includes several significant human pathogens, including the causative agent of botulism and an important cause of diarrhoea, Clostridium difficile. They are obligate anaerobes capable of producing endospores. [↩]
- Any lignan that is formed from another by metabolism in the gut. [↩]