The single layer of cells that line our gut has a total surface area larger than a tennis court. If this delicate single-cell layer is compromised, our health very soon deteriorates – with potentially fatal consequences. The unsung hero responsible for protecting our intestinal health is a chemical called butyrate. Let’s take a quick look at just how important this little-known short-chain fatty acid (SCFA) is and why dietary fibre is so vital to its functions.
There are literally trillions of bacteria in our intestines (also called the gut) – some good guys and some very bad guys. What we want to do is ensure that the good guys survive and the bad guys (such as campylobacter or salmonella) don’t.
But our bodies can mistake the good guys for bad guys if there’s not enough fibre in the diet – an increasingly common reality in the modern western diet of highly processed, low-fibre food.
Blog Contents
Good bacteria produce butyrate
Good bacteria in the colon feed on fibre and produce butyrate as a by-product. The butyrate in turn “calms down” our immune system, preventing it from regarding the good bacteria as foreign invaders and hence attacking them. However, if there’s insufficient buyrate being produced by the good bacteria – something that happens if they don’t have enough fibre to feed on – the immune system then assumes that the good bacteria are bad guys and attacks them. This can result in inflammation and the potential breakdown of the intestinal wall.
Quid Pro Quo
Research has demonstrated clearly that when we feed the good bacteria in our gut, they feed us right back; but stop feeding them and the health of our gut will deteriorate rapidly.
There’s an obvious evolutionary advantage for the good bacteria to want to keep us alive and healthy: if we die, they die.
This isn’t the case for the bad bacteria (such as cholera) which cause diarrhoea. The result of this is that they spread out of our bodies and can infect other people. It makes no difference to the bad guys whether we live or die – so long as we produce enough diarrhoea to spread them about the environment before we die.
A Fine Balance
Our immune systems have to maintain a fine balance between tolerating the good bacteria while attacking the bad. If this balance is disturbed by a lack of dietary fibre, it may lead to inflammatory bowel disease. Researchers found that butyrate “may behave as a microbial signal to inform [our] immune system that the relative levels of [good] bacteria are within the desired range.” If the butyrate levels are low, our immune system starts to attack all the gut bacteria.
Evolution vs Low-Fibre Diets
Butyrate has been involved in suppressing our immune systems for a very long time and for a very good reason. There are times when our guts are invaded by large amounts of bad bacteria. When this happens, we don’t want our immune system to go to sleep on the job – we want it to attack them and get rid of them. Once it’s done its job, and the bad guys are gone, the good bacteria will start producing butyrate and this will put the immune system back on “stand by”.
But this will only happen if we eat enough fibre; otherwise, our immune system stays on “red alert” with the resulting bad news for our intestinal health.
Fibre From Food Not supplements
Whilst research shows that fibre intake is “critical for optimal health”, there’s no magic pill that will replace the role played by the fibre in whole plant food. True, you come across bold claims for the effectiveness of fibre supplements such as Metamucil and psyllium, but the above research suggests that these supplements do “not replicate the results seen with a diet naturally high in fib[re].” This opinion is supported by Dr Greger.
The Many Health Benefits of Butyrate
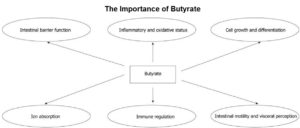
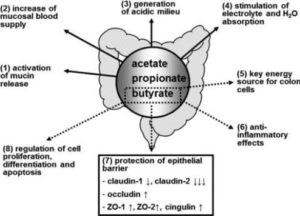
Researchers concluded that “[t]he effects exerted by butyrate are multiple and involve several distinct mechanisms of action.” as the main end product of the microbial fermentation of dietary fibres in the human intestine, butyrate plays a vital role by:
- maintaining intestinal homeostasis and overall health status
- regulating gene expression
- controlling the fate of cells through the inhibition of histone deacetylases (HDACs)
At the intestinal level, butyrate:
- prevents and inhibits colonic carcinogenesis
- protects against inflammation
- reduces oxidative stress
- provides a defence barrier for the epithelial cell layer
- modulates visceral sensitivity
- facilitates intestinal motility
At the extraintestinal level, butyrate offers potential for the treatment of:
- sickle cell disease
- β-thalassemia
- cystic fibrosis
- urea cycle enzyme deficiency
- X-linked adrenoleukodystrophy
- hypercholesterolemia
- obesity
- insulin resistance
- ischaemic stroke
Fork vs Pill
A growing number of studies (see References below for a wide variety of related research papers) have revealed new mechanisms and effects of butyrate. The majority of research projects are, of course, working within the paradigm that requires reductionist methods to produce patentable outcomes (largely pharmaceutical in nature). And whilst the evidence suggests that the fork is more effective than the pill in treating chronic non-communicable diseases (diabetes, hypertension, cancer, heart disease etc), the very fact that the scientific community is so positive about the wide range of benefits of butyrate – from the intestinal tract to peripheral tissues – reminds me of how wonderful the body is at healing itself. However, in order to ensure we benefit from the full protection of butyrate, we need to eat a WFPB diet packed full of fibre.
References
- Michael Greger M.D. FACLM. Prunes vs. Metamucil vs. Vegan Diet (Video). Nutritionfacts.org. March 15th, 2013 Volume 12
- James M. Harig, M.D., M.S., Konrad H. Soergel, M.D., Richard A. Komorowski, M.D., and Carol M. Wood, B.S.. Treatment of Diversion Colitis with Short-Chain-Fatty Acid Irrigation. January 5, 1989
N Engl J Med 1989; 320:23-28. DOI: 10.1056/NEJM198901053200105 - Shiu-Ming Kuo. The Interplay Between Fiber and the Intestinal Microbiome in the Inflammatory Response. Advances in Nutrition, Volume 4, Issue 1, 1 January 2013, Pages 16–28
- ttps://doi.org/10.3945/an.112.003046Pamela V. Chang, Liming Hao, Stefan Offermanns and Ruslan Medzhitov. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. PNAS February 11, 2014. 111 (6) 2247-2252; https://doi.org/10.1073/pnas.1322269111
- A. Attaluri, R. Donahoe, J. Valestin, K. Brown, S. S. C. Rao. Randomised clinical trial: Dried plums (prunes) vs. Psyllium for constipation. Aliment. Pharmacol. Ther. 2011 33(7):822 – 828
- V. Stanghellini, R. F. Cogliandro. Dried plums vs. psyllium. Aliment. Pharmacol. Ther. 2011 33(10):1180 – 1 – author – reply – 1181 – 2
- M. A. Sanjoaquin, P. N. Appleby, E. A. Spencer, T. J. Key. Nutrition and lifestyle in relation to bowel movement frequency: a cross sectional study of 20 630 men and women in EPIC-Oxford. Public Health Nutrition 2004 7(1):77-83
- E. P. Halmos, P. R. Gibson. Dried plums, constipation and the irritable bowel syndrome. Aliment Pharmacol Ther. 2011 Aug;34(3):396-7; author reply 397-8. doi: 10.1111/j.1365-2036.2011.04719.x
- J. W. McRorie. Prunes vs. psyllium for chronic idiopathic constipation. Aliment Pharmacol Ther. 2011 Jul;34(2):258-9. doi: 10.1111/j.1365-2036.2011.04713.x
- S. Lau, C. F. Donnellan, A. C. Ford. Do dried plums really help constipation? Aliment Pharmacol Ther. 2011 Jun;33(11):1258-9; author reply 1259. doi: 10.1111/j.1365-2036.2011.04649.x
- Michael Greger M.D. FACLM. The The Best Kept Secret in Medicine. Nutritionfacts.org. July 1st, 2015 Volume 25
- M A Hyman. The ecology of eating: the power of the fork. Altern Ther Health Med. 2009 Jul-Aug;15(4):14-5
- N D Barnard. The physician’s role in nutrition-related disorders: from bystander to leader. Virtual Mentor. 2013 Apr 1;15(4):367-72. doi: 10.1001/virtualmentor.2013.15.4.oped1-1304
- M A Hyman. The failure of risk factor treatment for primary prevention of chronic disease. Altern Ther Health Med. 2010 May-Jun;16(3):60-3
- I Shai, D Erlich, A D Cohen, M Urbach, N Yosef, O Levy, D R Shahar. The effect of personal lifestyle intervention among health care providers on their patients and clinics; the Promoting Health by Self Experience (PHASE) randomized controlled intervention trial. Prev Med. 2012 Oct;55(4):285-91.
- oi: 10.1016/j.ypmed.2012.08.001.
E Frank, J Breyan, L Elon. Physician disclosure of healthy personal behaviors improves credibility and ability to motivate. Arch Fam Med. 2000 Mar;9(3):287-90 - M W Kreuter, S G Chheda, F C Bull. How does physician advice influence patient behavior? Evidence for a priming effect. Arch Fam Med. 2000 May;9(5):426-33
- M A Kadoch. The power of nutrition as medicine. Prev Med. 2012 Jul;55(1):80. doi: 10.1016/j.ypmed.2012.04.013
- L Lianov, M Johnson. Physician competencies for prescribing lifestyle medicine. JAMA. 2010 Jul 14;304(2):202-3. doi: 10.1001/jama.2010.903
- M Ezzati, E Riboli. Can noncommunicable diseases be prevented? Lessons from studies of populations and individuals. Science. 2012 Sep 21;337(6101):1482-7
- Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23:366–384. [PubMed]
- Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. [PubMed]
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. [PubMed]
- Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297–313. [PubMed]
- Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. [PubMed]
- Binder HJ, Mehta P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology. 1989;96:989–996. [PubMed]
- Ramakrishna BS, Venkataraman S, Srinivasan P, Dash P, Young GP, Binder HJ. Amylase-resistant starch plus oral rehydration solution for cholera. N Engl J Med. 2000;342:308–313. [PubMed]
- Rabbani GH, Albert MJ, Rahman H, Chowdhury AK. Short-chain fatty acids inhibit fluid and electrolyte loss induced by cholera toxin in proximal colon of rabbit in vivo. Dig Dis Sci. 1999;44:1547–1553.[PubMed]
- Rabbani GH, Teka T, Zaman B, Majid N, Khatun M, Fuchs GJ. Clinical studies in persistent diarrhea: dietary management with green banana or pectin in Bangladeshi children. Gastroenterology. 2001;121:554–560. [PubMed]
- Alam NH, Ashraf H. Treatment of infectious diarrhea in children. Paediatr Drugs. 2003;5:151–165.[PubMed]
- Berni Canani R, Terrin G, Cirillo P, Castaldo G, Salvatore F, Cardillo G, Coruzzo A, Troncone R. Butyrate as an effective treatment of congenital chloride diarrhea. Gastroenterology. 2004;127:630–634.[PubMed]
- Wedenoja S, Holmberg C, Höglund P. Oral butyrate in treatment of congenital chloride diarrhea. Am J Gastroenterol. 2008;103:252–254. [PubMed]
- Kere J, Höglund P. Inherited disorders of ion transport in the intestine. Curr Opin Genet Dev. 2000;10:306–309. [PubMed]
- Roomans GM. Pharmacological approaches to correcting the ion transport defect in cystic fibrosis. Am J Respir Med. 2003;2:413–431. [PubMed]
- Chernova MN, Jiang L, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL. Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J Physiol. 2003;549:3–19. [PMC free article] [PubMed]
- Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, et al. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6:343–350.[PMC free article] [PubMed]
- Höglund P, Sormaala M, Haila S, Socha J, Rajaram U, Scheurlen W, Sinaasappel M, de Jonge H, Holmberg C, Yoshikawa H, et al. Identification of seven novel mutations including the first two genomic rearrangements in SLC26A3 mutated in congenital chloride diarrhea. Hum Mutat. 2001;18:233–242.[PubMed]
- Gadsby DC, Nairn AC. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol Rev. 1999;79:S77–S107. [PubMed]
- Rowe SM, Varga K, Rab A, Bebok Z, Byram K, Li Y, Sorscher EJ, Clancy JP. Restoration of W1282X CFTR activity by enhanced expression. Am J Respir Cell Mol Biol. 2007;37:347–56. [PMC free article][PubMed]
- Clausen MR, Bonnén H, Tvede M, Mortensen PB. Colonic fermentation to short-chain fatty acids is decreased in antibiotic-associated diarrhea. Gastroenterology. 1991;101:1497–1504. [PubMed]
- Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361:1496–1501.[PubMed]
- Burkitt DP. Epidemiology of cancer of the colon and rectum. 1971. Dis Colon Rectum. 1993;36:1071–1082. [PubMed]
- Cassidy A, Bingham SA, Cummings JH. Starch intake and colorectal cancer risk: an international comparison. Br J Cancer. 1994;69:937–942. [PMC free article] [PubMed]
- Howe GR, Benito E, Castelleto R, Cornée J, Estève J, Gallagher RP, Iscovich JM, Deng-ao J, Kaaks R, Kune GA. Dietary intake of fiber and decreased risk of cancers of the colon and rectum: evidence from the combined analysis of 13 case-control studies. J Natl Cancer Inst. 1992;84:1887–1896. [PubMed]
- Kim YI. AGA technical review: impact of dietary fiber on colon cancer occurrence. Gastroenterology. 2000;118:1235–1257. [PubMed]
- Park Y, Hunter DJ, Spiegelman D, Bergkvist L, Berrino F, van den Brandt PA, Buring JE, Colditz GA, Freudenheim JL, Fuchs CS, et al. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. JAMA. 2005;294:2849–2857. [PubMed]
- Comalada M, Bailón E, de Haro O, Lara-Villoslada F, Xaus J, Zarzuelo A, Gálvez J. The effects of short-chain fatty acids on colon epithelial proliferation and survival depend on the cellular phenotype. J Cancer Res Clin Oncol. 2006;132:487–497. [PubMed]
- Hodin RA, Meng S, Archer S, Tang R. Cellular growth state differentially regulates enterocyte gene expression in butyrate-treated HT-29 cells. Cell Growth Differ. 1996;7:647–653. [PubMed]
- Chirakkal H, Leech SH, Brookes KE, Prais AL, Waby JS, Corfe BM. Upregulation of BAK by butyrate in the colon is associated with increased Sp3 binding. Oncogene. 2006;25:7192–7200. [PubMed]
- Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr. 2002;132:1012–1017. [PubMed]
- Alrawi SJ, Schiff M, Carroll RE, Dayton M, Gibbs JF, Kulavlat M, Tan D, Berman K, Stoler DL, Anderson GR. Aberrant crypt foci. Anticancer Res. 2006;26:107–119. [PubMed]
- Scharlau D, Borowicki A, Habermann N, Hofmann T, Klenow S, Miene C, Munjal U, Stein K, Glei M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. 2009;682:39–53. [PubMed]
- Gibson PR, Rosella O, Wilson AJ, Mariadason JM, Rickard K, Byron K, Barkla DH. Colonic epithelial cell activation and the paradoxical effects of butyrate. Carcinogenesis. 1999;20:539–544. [PubMed]
- Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S–2493S.[PubMed]
- Kouraklis G, Theocharis S. Histone deacetylase inhibitors: a novel target of anticancer therapy (review). Oncol Rep. 2006;15:489–494. [PubMed]
- Chen YX, Fang JY, Lu J, Qiu DK. Regulation of histone acetylation on the expression of cell cycle-associated genes in human colon cancer cell lines. Zhonghua Yixue Zazhi. 2004;84:312–317. [PubMed]
- Yu DC, Waby JS, Chirakkal H, Staton CA, Corfe BM. Butyrate suppresses expression of neuropilin I in colorectal cell lines through inhibition of Sp1 transactivation. Mol Cancer. 2010;9:276. [PMC free article][PubMed]
- Ruemmele FM, Dionne S, Qureshi I, Sarma DS, Levy E, Seidman EG. Butyrate mediates Caco-2 cell apoptosis via up-regulation of pro-apoptotic BAK and inducing caspase-3 mediated cleavage of poly-(ADP-ribose) polymerase (PARP) Cell Death Differ. 1999;6:729–735. [PubMed]
- Ruemmele FM, Schwartz S, Seidman EG, Dionne S, Levy E, Lentze MJ. Butyrate induced Caco-2 cell apoptosis is mediated via the mitochondrial pathway. Gut. 2003;52:94–100. [PMC free article] [PubMed]
- Soga T, Kamohara M, Takasaki J, Matsumoto S, Saito T, Ohishi T, Hiyama H, Matsuo A, Matsushime H, Furuichi K. Molecular identification of nicotinic acid receptor. Biochem Biophys Res Commun. 2003;303:364–369. [PubMed]
- Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, Ignar DM, Murdock PR, Steplewski K, Green A, et al. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem. 2003;278:9869–9874. [PubMed]
- Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832.[PMC free article] [PubMed]
- Bordonaro M, Lazarova DL, Sartorelli AC. Butyrate and Wnt signaling: a possible solution to the puzzle of dietary fiber and colon cancer risk? Cell Cycle. 2008;7:1178–1183. [PubMed]
- Scheppach W, Weiler F. The butyrate story: old wine in new bottles? Curr Opin Clin Nutr Metab Care 2004; 7: 563-567 [PubMed]
- Pool-Zobel B, Veeriah S, Böhmer FD. Modulation of xenobiotic metabolising enzymes by anticarcinogens-focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat Res. 2005;591:74–92. [PubMed]
- Scharlau D, Borowicki A, Habermann N, Hofmann T, Klenow S, Miene C, Munjal U, Stein K, Glei M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. 2009;682:39–53. [PubMed]
- Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118:724–734. [PubMed]
- Jobin C, Sartor RB. The I kappa B/NF-kappa B system: a key determinant of mucosalinflammation and protection. Am J Physiol Cell Physiol. 2000;278:C451–C462. [PubMed]
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. [PubMed]
- Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. [PubMed]
- Shao J, Fujiwara T, Kadowaki Y, Fukazawa T, Waku T, Itoshima T, Yamatsuji T, Nishizaki M, Roth JA, Tanaka N. Overexpression of the wild-type p53 gene inhibits NF-kappaB activity and synergizes with aspirin to induce apoptosis in human colon cancer cells. Oncogene. 2000;19:726–736. [PubMed]
- Lind DS, Hochwald SN, Malaty J, Rekkas S, Hebig P, Mishra G, Moldawer LL, Copeland EM 3rd, Mackay S. Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery. 2001;130:363–369.[PubMed]
- Segain JP, Raingeard de la Blétière D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottière HM, Galmiche JP. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47:397–403. [PMC free article] [PubMed]
- Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, Knuechel R, Baeuerle PA, Schölmerich J, Gross V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. [PubMed]
- Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. [PMC free article] [PubMed]
- Schwab M, Reynders V, Loitsch S, Steinhilber D, Stein J, Schröder O. Involvement of different nuclear hormone receptors in butyrate-mediated inhibition of inducible NF kappa B signalling. Mol Immunol. 2007;44:3625–3632. [PubMed]
- Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L. Inhibition of interferon gamma signaling by the short chain fatty acid butyrate. Mol Cancer Res. 2003;1:855–862. [PubMed]
- Meijer K, de Vos P, Priebe MG. Butyrate and other short-chain fatty acids as modulators of immunity: what relevance for health? Curr Opin Clin Nutr Metab Care. 2010;13:715–721. [PubMed]
- Sina C, Gavrilova O, Förster M, Till A, Derer S, Hildebrand F, Raabe B, Chalaris A, Scheller J, Rehmann A, et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol. 2009;183:7514–7522. [PubMed]
- Hallert C, Björck I, Nyman M, Pousette A, Grännö C, Svensson H. Increasing fecal butyrate in ulcerative colitis patients by diet: controlled pilot study. Inflamm Bowel Dis. 2003;9:116–121. [PubMed]
- Vernia P, Annese V, Bresci G, d’Albasio G, D’Incà R, Giaccari S, Ingrosso M, Mansi C, Riegler G, Valpiani D, et al. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur J Clin Invest. 2003;33:244–248. [PubMed]
- Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, Richter F, Dusel G, Kasper H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103:51–56. [PubMed]
- Lührs H, Gerke T, Müller JG, Melcher R, Schauber J, Boxberge F, Scheppach W, Menzel T. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. 2002;37:458–466. [PubMed]
- Vernia P, Marcheggiano A, Caprilli R, Frieri G, Corrao G, Valpiani D, Di Paolo MC, Paoluzi P, Torsoli A. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment Pharmacol Ther. 1995;9:309–313. [PubMed]
- Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis. 2010;16:684–695. [PubMed]
- Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci. 2007;52:2015–2021. [PubMed]
- Skrzydlewska E, Sulkowski S, Koda M, Zalewski B, Kanczuga-Koda L, Sulkowska M. Lipid peroxidation and antioxidant status in colorectal cancer. World J Gastroenterol. 2005;11:403–406.[PMC free article] [PubMed]
- Hamer HM, Jonkers DM, Bast A, Vanhoutvin SA, Fischer MA, Kodde A, Troost FJ, Venema K, Brummer RJ. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr. 2009;28:88–93. [PubMed]
- Hatayama H, Iwashita J, Kuwajima A, Abe T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem Biophys Res Commun. 2007;356:599–603. [PubMed]
- Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. [PMC free article] [PubMed]
- Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, Ohtake T, Obonyo M, Gallo RL, Eckmann L, Kagnoff MF. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology. 2003;125:1613–1625. [PubMed]
- Kida Y, Shimizu T, Kuwano K. Sodium butyrate up-regulates cathelicidin gene expression via activator protein-1 and histone acetylation at the promoter region in a human lung epithelial cell line, EBC-1. Mol Immunol. 2006;43:1972–1981. [PubMed]
- Schauber J, Iffland K, Frisch S, Kudlich T, Schmausser B, Eck M, Menzel T, Gostner A, Lührs H, Scheppach W. Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol Immunol. 2004;41:847–854. [PubMed]
- Schauber J, Svanholm C, Termén S, Iffland K, Menzel T, Scheppach W, Melcher R, Agerberth B, Lührs H, Gudmundsson GH. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52:735–741. [PMC free article] [PubMed]
- Steinmann J, Halldórsson S, Agerberth B, Gudmundsson GH. Phenylbutyrate induces antimicrobial peptide expression. Antimicrob Agents Chemother. 2009;53:5127–5133. [PMC free article] [PubMed]
- Mariadason JM, Barkla DH, Gibson PR. Effect of short-chain fatty acids on paracellular permeability in Caco-2 intestinal epithelium model. Am J Physiol. 1997;272:G705–G712. [PubMed]
- Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61:37–41. [PubMed]
- Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–1625. [PMC free article] [PubMed]
- Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–1782. [PubMed]
- Vanhoutvin SA, Troost FJ, Kilkens TO, Lindsey PJ, Hamer HM, Jonkers DM, Venema K, Brummer RJ. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil. 2009;21:952–976. [PubMed]
- Kilkens TO, Honig A, van Nieuwenhoven MA, Riedel WJ, Brummer RJ. Acute tryptophan depletion affects brain-gut responses in irritable bowel syndrome patients and controls. Gut. 2004;53:1794–1800.[PMC free article] [PubMed]
- Chen PS, Wang CC, Bortner CD, Peng GS, Wu X, Pang H, Lu RB, Gean PW, Chuang DM, Hong JS. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience. 2007;149:203–212.[PMC free article] [PubMed]
- Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–R1276. [PubMed]
- Mitsui R, Ono S, Karaki S, Kuwahara A. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol Motil. 2005;17:585–594. [PubMed]
- Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. 2008;59 Suppl 2:251–262.[PubMed]
- Atweh GF, Sutton M, Nassif I, Boosalis V, Dover GJ, Wallenstein S, Wright E, McMahon L, Stamatoyannopoulos G, Faller DV, et al. Sustained induction of fetal hemoglobin by pulse butyrate therapy in sickle cell disease. Blood. 1999;93:1790–1797. [PMC free article] [PubMed]
- Perrine SP, Ginder GD, Faller DV, Dover GH, Ikuta T, Witkowska HE, Cai SP, Vichinsky EP, Olivieri NF. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders. N Engl J Med. 1993;328:81–86. [PubMed]
- Collins AF, Pearson HA, Giardina P, McDonagh KT, Brusilow SW, Dover GJ. Oral sodium phenylbutyrate therapy in homozygous beta thalassemia: a clinical trial. Blood. 1995;85:43–49. [PubMed]
- Dover GJ, Brusilow S, Charache S. Induction of fetal hemoglobin production in subjects with sickle cell anemia by oral sodium phenylbutyrate. Blood. 1994;84:339–343. [PubMed]
- Perrine SP, Rudolph A, Faller DV, Roman C, Cohen RA, Chen SJ, Kan YW. Butyrate infusions in the ovine fetus delay the biologic clock for globin gene switching. Proc Natl Acad Sci USA. 1988;85:8540–8542. [PMC free article] [PubMed]
- Weinberg RS, Ji X, Sutton M, Perrine S, Galperin Y, Li Q, Liebhaber SA, Stamatoyannopoulos G, Atweh GF. Butyrate increases the efficiency of translation of gamma-globin mRNA. Blood. 2005;105:1807–1809. [PMC free article] [PubMed]
- Mabaera R, West RJ, Conine SJ, Macari ER, Boyd CD, Engman CA, Lowrey CH. A cell stress signaling model of fetal hemoglobin induction: what doesn’t kill red blood cells may make them stronger. Exp Hematol. 2008;36:1057–1072. [PubMed]
- Burlina AB, Ogier H, Korall H, Trefz FK. Long-term treatment with sodium phenylbutyrate in ornithine transcarbamylase-deficient patients. Mol Genet Metab. 2001;72:351–355. [PubMed]
- Kemp S, Wei HM, Lu JF, Braiterman LT, McGuinness MC, Moser AB, Watkins PA, Smith KD. Gene redundancy and pharmacological gene therapy: implications for X-linked adrenoleukodystrophy. Nat Med. 1998;4:1261–1268. [PubMed]
- Gylling H. Cholesterol metabolism and its implications for therapeutic interventions in patients with hypercholesterolaemia. Int J Clin Pract. 2004;58:859–866. [PubMed]
- Sviridov DD, Safonova IG, Talalaev AG, Repin VS, Smirnov VN. Regulation of cholesterol synthesis in isolated epithelial cells of human small intestine. Lipids. 1986;21:759–763. [PubMed]
- Alvaro A, Solà R, Rosales R, Ribalta J, Anguera A, Masana L, Vallvé JC. Gene expression analysis of a human enterocyte cell line reveals downregulation of cholesterol biosynthesis in response to short-chain fatty acids. IUBMB Life. 2008;60:757–764. [PubMed]
- Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. [PMC free article][PubMed]
- Kim HJ, Leeds P, Chuang DM. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem. 2009;110:1226–1240. [PMC free article] [PubMed]
- Thomas DW, Greer FR. Probiotics and prebiotics in pediatrics. Pediatrics. 2010;126:1217–1231.[PubMed]
- Jacobasch G, Schmiedl D, Kruschewski M, Schmehl K. Dietary resistant starch and chronic inflammatory bowel diseases. Int J Colorectal Dis. 1999;14:201–211. [PubMed]
- Stein TP, Yoshida S, Schluter MD, Drews D, Assimon SA, Leskiw MJ. Comparison of intravenous nutrients on gut mucosal proteins synthesis. JPEN J Parenter Enteral Nutr. 1994;18:447–452. [PubMed]
- Tappenden KA. Emerging therapies for intestinal failure. Arch Surg. 2010;145:528–532. [PubMed]
- Goldstein NS. Serrated pathway and APC (conventionUse)-type colorectal polyps: molecular-morphologic correlations, genetic pathways, and implications for classification. Am J Clin Pathol. 2006;125:146–153. [PubMed]
- Gassull MA. Review article: the intestinal lumen as a therapeutic target in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24 Suppl 3:90–95. [PubMed]
- Diakos C, Prieschl EE, Säemann MD, Böhmig GA, Csonga R, Sobanov Y, Baumruker T, Zlabinger GJ. n-Butyrate inhibits Jun NH(2)-terminal kinase activation and cytokine transcription in mast cells. Biochem Biophys Res Commun. 2006;349:863–868. [PubMed]